Programme directors
Associate Professor Haizal Hussaini, Programme leader
Associate Professor Lara Friedlander, Deputy programme leader
Programme staff
Key personnel, postgraduate students and collaborations
Understanding diseases in order to improve diagnosis and treatment
The aim of our research group is to investigate the cellular, and molecular basis of oral diseases, and their treatment. We focus our work in four areas:
- Oral mucosal disease (including oral lichen planus and squamous cell carcinoma)
- Cellular stress pathway
- Angiogenesis and tissue regeneration
- Immune pulp response and regeneration
Immunopathological mechanisms underpinning oral mucosal diseases
Microenvironment of oral cancer
We are investigating various factors associated with oral squamous cell carcinoma (OSCC), to develop a greater understanding of the tumour microenvironment. This includes assessment of the tumour cells themselves, but we are particularly interested in the communication between tumour cells, local immune cells and vascular and lymphatic cells.
Regulation of immune responses in oral diseases
OSCC develops in an immune cell-rich environment, where inflammatory cells in the tumour microenvironment establish an anti-tumour response by secreting pro-inflammatory cytokines. At the same time the cancer cells may induce various mechanisms suppressing the anti-tumour response such as regulating a network of suppressive cytokines and the recruitment of suppressive Tregs. These escape mechanisms are seen at the local tumour site and similar mechanisms may also occur in regional lymph nodes. We are currently investigating the concept that the escape of malignant oral keratinocytes from the primary site, and their metastasis to regional lymph nodes, is orchestrated by regulatory T cells and their associated immune repertoire.
Oral lichen planus and immunotherapy
Oral lichen planus (OLP) is a common chronic immune-mediated disease. We are comparing the number of cells expressing various immune markers in OLP with non-specifically inflamed oral mucosa using immunohistochemistry and determining gene expression with quantitative real-time reverse transcriptase polymerase chain reaction. We are currently investigatingt the potential of using immunotherapy for the treatment of OLP with in vitro investigations, supported by funding from industry.
Endoplasmic reticulum stress pathway in oral diseases
Cellular stress pathways known as the unfolded protein response (UPR) are activated when the endoplasmic reticulum, the protein-producing factory within the cell, is stressed. ER stress modulates UPR pathways, thus partially determining the cellular responses to disease. Evidence suggests that UPR components are activated to either inhibit cancer growth or promote its progression and this is being investigated in a series of projects. The affect on UPR in periodontal ligament cells subjected to mechanical strain, mimicking orthodontic tooth movement, is a further area of study.
Angiogenesis and lymphangiogenesis
In oral cancer
We have shown that angiogenic factors were expressed on epithelial cells as well as endothelial cells in OSCC. These findings offer an insight into upregulation of pro-angiogenic genes in oral cancer. In the future, anti-angiogenic therapies in OSCC could prove to be useful as an adjunct to conventional surgical and chemotherapeutic treatments.
Dentine-pulp development
We are investigating angiogenesis, growth factor and cytokine activity in pulp biology in relation to pulp-dentine development and response to injury. In particular, the apex of immature permanent teeth has been shown to contain a rich source of angiogenic factors which may contribute to development and healing. The impact of Type 2 Diabetes on the dental pulp is a further area of investigation and our findings indicate that there are distinct histological and immunological differences in the pulps of healthy and diabetic patients. This is clinically significant and may suggest that there are similar processes happening in dental tissues to elsewhere in the body.
Immune pulp response and regeneration
Dental caries is a prevalent chronic disease that affects one in three adults in New Zealand. Caries progression destroys tooth enamel and dentine and, if left untreated, will lead to dental pulp infections and significant pain. Pulp infection results in uncontrolled inflammation, detrimental for tooth regeneration and healing. Mineral Trioxide aggregate (MTA) and calcium hydroxide are currently the mainstay therapies for infection and inflammation in vital pulp. Currently we are investigating a new immunotherapeutic molecule that can modulate pulp inflammation and allowing pulp regeneration to occur, retaining tooth vitality and prevent tooth loss. This research is currently funded by the Health Research Council of New Zealand.
More information
Our Oral Molecular and Immunopathology programme profile (linked at right) contains more details on our current research projects, collaborations funding highlights and recent publications.
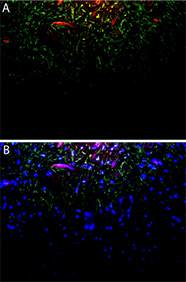 Double Immunofluorescence with vimentin and RAGE (receptor for advanced glycation end products) in the cell rich zone of the coronal pulp showing expression on fibroblasts which are positive for vimentin. Elsewhere in the body, RAGE leads to pro-inflammatory gene activation and is elevated in diabetes. From the PhD research of Shaikha Al Samahi.
Double Immunofluorescence with vimentin and RAGE (receptor for advanced glycation end products) in the cell rich zone of the coronal pulp showing expression on fibroblasts which are positive for vimentin. Elsewhere in the body, RAGE leads to pro-inflammatory gene activation and is elevated in diabetes. From the PhD research of Shaikha Al Samahi.
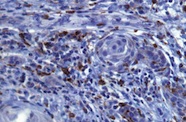
Macrophages reacting with CD68 in the stroma adjacent to infiltrating squamous cell carcinoma islands. Avadhoot Avadhani's PhD project showing an assessment of the immune response in oral cancer.
More information
Oral molecular and immunopathology research programme profile
(Extract from our 2019-2020 SJWRI Research Report)
Key research staff, postgraduate students and collaborations
