6.1. Overview of Module 6
This module provides guidance on intravenous vitamin C (IVC) administration for practitioners. It provides what you need to know about prescribing and managing care, including protocols for the two main types of treatment programmes (immune support and support for people with cancer). Later sections cover delivering treatment including preparing solutions and managing any problems during administration.
Following completion of this module the participant will be able to:
- Discuss with patients the evidence-based role of vitamin C administration for immune support and support of patients with cancer (Communication, Scholarship, Professionalism)
- Assess patient risk factors and safety of vitamin C administration in clinical practice (Clinical expertise)
- Discuss with patients the possible side-effects of IV vitamin C administration (Communication, Scholarship, Professionalism)
- Apply knowledge on vitamin C administration to patients in their healthcare practice (Clinical expertise)
Length of activity: 25 min reading
RNZCGP Endorsed: 0.4 credits
CICM Accredited: 0.4 points.
6.2. Introduction
IVC treatment should be carried out by registered clinical practitioners.
This module explains how doctors and nurses at Integrated Health Options (“The Clinic”) in Auckland, New Zealand, conducted IVC treatment for their patients based on scientific evidence and many years of clinical experience (1981–2020).
It is the responsibility of all healthcare practitioners referring to these guidelines to adapt them for safe use within their practice and for the individual needs of their patients.
6.3. Managing care
6.3.1. Prescribing overview
Medicine safety
Ascor L 500® by McGuff Pharmaceuticals is currently the only IVC product that has MedSafe approval in New Zealand. Sodium Ascorbate Solution by Biological Therapies is available in Australia. These are manufactured and distributed to pharmaceutical standards. Please refer to the product safety datasheets (see additional resources below).
Additional resources
Intravenous vitamin C treatment programmes
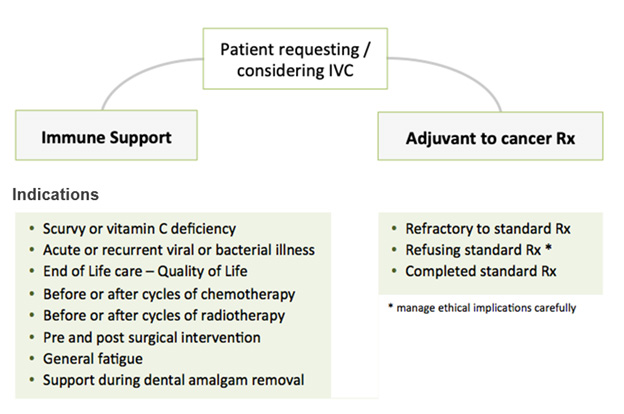
There are two broad programmes of intravenous vitamin C (IVC) treatment you might prescribe and manage:
- Immune support.
- Support for people with cancer.
See the IVC Treatment Protocols section below for details.
Indications, contra-indications, precautions
This section is a summary. For more detail, refer to the rest of this guideline document.
Interactions with other treatments and investigations
IVC can interact with some other treatments and investigations. Refer to the Stand-down Times section below.
Assessing risk
When assessing the risk level of IVC treatment for a patient, consider both clinical and ethical factors, including but not limited to:
| Low risk | Riskier | |
|---|---|---|
| Clinical | Ethical | |
|
|
|
6.3.2. Managing intravenous vitamin C therapy
Indications
Vitamin C infusions are indicated for:
- Scurvy (National Institutes of Health, 2016).
- Vitamin C insufficiency.
Vitamin C infusions are also given for:
- Viral/Bacterial infections (Ströhle, et al., 2011).
- Cancer (Fritz, et al., 2014).
- Post-operative wellbeing (Baker, et al., 2016; Ayatollahi, et al., 2016).
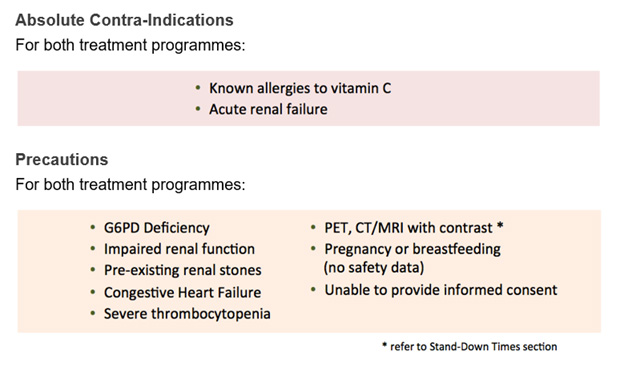
Contra-indications
- Acute renal failure.
- Previous allergic reaction to vitamin C administration.
Treatment precautions
- Some people have a genetic deficiency that means higher doses of vitamin C may cause haemolysis (Rees, et al., 1993). Testing all patient's G6PD levels is strongly recommended before giving any more than 25g of Ascorbic Acid or 30g of Sodium Ascorbate.
- Patients at risk of fluid or sodium overload – such as with congestive heart failure, pedal and/or sacral oedema, acute and/or chronic renal failure, or dehydration – should be carefully managed. (Mikirova, et al., 2013).
- Use of IVC in patients who are undergoing chemotherapy or radiotherapy should be discussed with the patient and their oncologist. Pre- and post- chemotherapy or radiotherapy may be advantageous (Ma, et al., 2014; Carr, et al., 2014). See the Stand-down Times section below for general guidance.
- Patients can become mildly dehydrated so ensure patients are well hydrated before and during the infusion.
- If the urine becomes deep orange/black – dipstick the urine to check for haemolysis. Ensure G6PD is normal.
- If blood test indicates hypercalcaemia, avoid supplemental calcium in the infusion or orally. Do not prescribe vitamin D.
- Although excessive oral vitamin C is not recommended in people with haemochromatosis, the effect of intravenous vitamin C has not been studied in this population. Clinical experience has not raised any concerns relating to IVC increasing iron stores in people with haemochromatosis. However, if IVC is administered to individuals with iron storage diseases, regular monitoring of iron status is recommended.
- Pregnancy or breastfeeding (no safety data).
- IVC can interact with some other treatments. Refer to Stand-down Times below.
Renal stones
- Oxalic acid is an end product of metabolic oxidation of vitamin C. Oxalate nephropathy has been reported after administration of IVC in subjects with renal dysfunction (Wong 1994, Cossey 2013). However, in people with normal renal function the risk of oxalate crystallization in the kidney was not increased (Robitaille 2009).
- Clinical experience is that patients do not experience an increased incidence of renal stones compared to the general population (Prier, M, et al., 2018). In patients with a past history of renal stones the risk should be discussed before administering IVC.
Testing precautions
- Leave at least 24 hours between IVC infusions and renal function testing to avoid false creatinine readings.
- For insulin-dependent patients who rely on test-strip readings for their insulin dose, there is a risk of overdose causing hypoglycaemia. Patients with diabetes should not rely on finger-prick (capillary) glucose tests until 8–10 hours after IVC treatment due to false elevation of readings when using test strips (Vasudevan, et al., 2014) – possibly even 12 hours after treatment.
NOTE: A laboratory blood serum glucose test is not affected (Jackson, et al., 2006). - IVC can interact with some investigations. Refer to Stand-down Times below.
Additional resources
References – 6.3.2. Managing intravenous vitamin C therapy (PDF)
6.3.3. IVC Treatment protocols
There are slightly different protocols for Immune Support and Cancer Support programmes, described below. They share recommendations for consultation, testing, treatment review, and injectable preparations.
Consultation
- Take a comprehensive history and examination focusing on presenting health concerns, family history, past history including any history of renal stones, medications, supplements, allergies and focused physical examination as appropriate.
- Record patient's current oral vitamin C dosage.
- Develop a treatment plan for the appropriate treatment programme below.
- Relevant to the patient's condition and needs, discuss how vitamin C may act, its safety, side effects, and potential benefits. Patient must sign a Consent for Treatment.
Testing
- It is strongly recommended that blood tests be ordered prior to commencing doses greater than 25g of Ascorbic Acid or 30g of Sodium Ascorbate. This should include a G6PD, Renal Function (Creatinine, eGFR, Electrolytes), Calcium, CRP, and FBC. See the Managing G6PD Deficiency section below.
- It is usually safe to give up to 25g of ascorbic acid or 30g of Sodium Ascorbate before G6PD test results are back.
- It is advised that renal function is monitored throughout treatment. Testing is usually conducted at commencement of treatment, at four weeks, twelve weeks, then every three months. Patients with lower baseline renal function may be tested more frequently. See the Managing Renal Function section below.
- Additional tests may be patient-specific and ordered at the discretion of the consulting doctor.
Treatment review
Treatment review is recommended at 2 weeks, 6 weeks, 3 months, 6 months or as indicated by patient needs.
Injectable preparations
Standard clinical practices apply around prescribing for intravenous administration, including taking osmolarity into account. The following is for guidance only, based on clinical experience.
Carrier
The carrier solution for administration can be 0.9% saline, sterile water for injection, or glucose 5%. The dosage of Ascorbic Acid/Sodium Ascorbate may affect which carrier is prescribed.
- Patients managing a cancer diagnosis may prefer to avoid glucose.
- For doses above 50g of Ascorbic Acid or Sodium Ascorbate, saline is not recommended as a carrier due to impact on osmolarity.
| Ascorbic Acid dose | Sodium Ascorbate dose | Carrier bag size | Saline 0.9% | Glucose 5% | Water for injection |
|---|---|---|---|---|---|
| <50g | <50g | 250ml | Yes | Yes | Yes |
| 50–100g | 50–90g | 500ml | No | No | Yes |
| >100g | >90g | 1,000ml | No | No | Yes |
Additives
- Ascorbic Acid 500 mg/mL or Sodium Ascorbate 300 mg/mL
- Ascorbic Acid 25g in 50mL.
- Sodium Ascorbate equivalent Ascorbic Acid, 26.5g in 100mL
- (Optional) Calcium Gluconate 10% – 1g in 10 mL
Immune support
Treatments are generally recommended 1 – 2 times per week but can be administered more frequently at the doctor's discretion and as indicated by patient needs.
1st treatment
- Generally, 15–25g Ascorbic Acid or 15–30g Sodium Ascorbate.
- Saline 0.9%, sterile water for injection, or glucose 5%: 250mL.
2nd and subsequent treatments
- 15–60g Sodium Ascorbate / 15–50g Ascorbic Acid.
- Saline 0.9%, sterile water for injection, or glucose 5%: 250mL.
Optional
- If previous symptomatic hypocalcaemia during infusion, consider 10mL Calcium Gluconate for a patient receiving 50g Ascorbic Acid or 60g Sodium Ascorbate or greater (1g/10mL), unless hypercalcaemic.
Support for people with cancer
- The usual treatment plan is two treatments per week for 6–8 weeks initially.
- If there is indication of benefit at review – including quality of life improvements, monitoring blood tests and any available scans - patient may continue with 1-2 treatments per week for another 6 weeks. If there is ongoing benefit, patient may continue treatment every 1 to 3 weeks longer-term. The administration of a standard EORTC QLQ-C30 questionnaire to monitor each patient's quality of life throughout treatment is recommended.
- If available, plasma vitamin C levels are tested once IVC dose is up to 1–1.1 g/kg of Ascorbic Acid or 1.1–1.2 g/kg of Sodium Ascorbate, until tolerance level is achieved. Plasma vitamin C levels are used to guide tolerance dosing. Aim for 350–400 mg/dL (Mikirova, et al., 2013; Chen, et al., 2015).
- Monitoring plasma vitamin C tests are conducted every 8–10 treatments to check tolerance levels are being maintained. Adjust IVC dose as required.
1st treatment
- Generally, 15–25g Ascorbic Acid or 15–30g Sodium Ascorbate.
- Saline 0.9% or sterile water for injection: 250mL.
2nd treatment
- 25–50g Ascorbic Acid or 30–60g Sodium Ascorbate.
- Saline 0.9% or sterile water for injection: 250 mL.
3rd and subsequent treatments
- Ascorbic Acid can be increased up to 1.1 g/kg for males, and 1 g/kg for females, as tolerated.
- Sodium Ascorbate can be increased up to 1.2 g/kg for males, and 1.1 g/kg for females, as tolerated.
- See the Preparing Intravenous Solutions section below for guidance on bag volumes.
Optional
- If previous symptomatic hypocalcaemia during infusion, consider 10mL Calcium Gluconate for a patient receiving 50g Ascorbic Acid or 60g Sodium Ascorbate or greater (1g/10mL), unless hypercalcaemic.
6.3.4. Consent for intravenous treatment
It is expected that each clinic will have its own approach to gaining the patient's written informed consent before beginning IVC treatment, reflecting its own professional obligations and advice. For your interest, this section presents one possible approach used at the Clinic.
How is consent understood?
An interactive process between health practitioner and patient
- Explain options.
- Expected risks, side effects, benefits and costs of proposed treatment.
- Convey information in form, language, manner suitable for patient.
- Requires:
- Trust.
- Non-pressured environment.
- Support people if wanted.
- Opportunity to ask questions/clarify/consider.
- Can take away information if want.
A patient right
Right to make an informed choice and to give informed consent.
“right to information that a reasonable consumer, in that consumer's circumstances, would expect to receive” (Information, choice of treatment and informed consent. Medical Council of NZ, March 2011).
Consent discussion – topics to cover
- Understanding IVC treatment for patient's condition.
- Discuss safety – any known concerns/issues.
- Discuss side effects.
- Discuss available evidence.
- Advise can withdraw consent at any time.
- Discuss cost.
- Check consent to communicate with other health providers.
- Advise that this treatment is not supported by the majority of doctors.
Additional resources
Consent form example and Consent discussion – example content (PDF)
6.3.5. Managing G6PD deficiency
Some people have a genetic deficiency which may cause haemolysis with high dose intravenous vitamin C (Campbell, et al., 1975 & Rees, et al., 1993). Testing the patient's G6PD level before giving any more than 25g of Ascorbic Acid or 30g of Sodium Ascorbate is strongly recommended.
This section describes:
- Testing for potential G6PD genetic deficiency.
- Managing the situation appropriately if the test result is below normal range.
Testing for G6PD deficiency
(Mehta, et al., 1990; Clinical Experience; Labtests reference range)
- Testing for G6PD deficiency is required for all patients considering having more than 25g of Ascorbic Acid or 30g of Sodium Ascorbate intravenously.
- Administering up to 25g of Ascorbic Acid or 30g of Sodium Ascorbate intravenously is considered safe in patients with G6PD deficiency.
- Normal range for G6PD is 7–20 U/g Hb.
If results are below 7 U/g Hb
(Clinical Experience; advice from Labtests pathologist)
- Repeat G6PD test.
- Notify the patient's regular GP (if not yourself) that they have G6PD deficiency.
- Avoid administering > 25g of Ascorbic Acid or 30g of Sodium Ascorbate intravenously.
- Refer patients to www.patient.co.uk or a similar source for further information on G6PD deficiency.
- If you have any concern of low-grade haemolysis, order a haemolytic screen shortly after IVC administration: CBC, serum bilirubin, serum LDH, reticulocyte count.
Additional resources
6.3.6. Managing renal function
Poor initial or unnoticed subsequent deterioration in patient renal function may affect ability to handle intravenous vitamin C (IVC) treatment. It is advised that the renal function of patients is monitored throughout a course of treatment and that any changes are managed.
Renal function testing
(Clinical Experience; Best Practice Guidelines)
- Obtain a baseline renal function test (RFT) on all new patients.
- For those having IVC, repeat RFT at 4 weeks, 12 weeks and then every 12 weeks.
Managing changes in renal function
(BPAC, 2015; Kidney Health New Zealand, 2013; Clinical Experience)
- If the eGFR reduces 15–20%, repeat the RFT in 1–2 weeks. If there is further reduction, stop IVC and review patient as below.
- If the drop is within normal range (eGFR > 60) and is stable at recheck, review patient for obvious cause. If examination and investigation are normal, use clinical judgement to decide whether to either:
- continue IVC with precaution of frequent RFT checks, or
- stop IVC until cause is found.
- If the eGFR reduces more than 20%, stop IVC, review the patient, address any possible causes (eg: dehydration, high meat intake before the test, test done within 24 hours of infusion, new medications, etc).
- Repeat RFT 1–2 weeks later. If normalised or improving, restart IVC and monitor more closely until stabilised. If level continues to reduce, do not restart IVC until cause found. Refer back to GP.
Additional resources
6.3.7. Stand-down times
This section specifies desirable intervals between intravenous vitamin C (IVC) treatment and other forms of medical treatment or investigation.
IVC excretion
Vitamin C is promptly excreted by the kidneys with a half-life of 2.0 ± 0.6 hours, so after an intravenous infusion blood levels of vitamin C return to normal after 8-10 hours (Stephenson, et al., 2013).
There are some effects which may cause confusion to clinicians particularly if they have not been informed that the patient is having IVC infusions.
Summary of stand-down periods
Because of potential interactions, a clear period is recommended between IVC and other treatments.
For example, if a patient is scheduled for Chemotherapy on a Wednesday, their previous IVC treatment is recommended to be no later than Monday.
| Clear period after IVC, before procedure | Procedure | Clear period after Procedure, before IVC |
|---|---|---|
| 24 hours | Blood tests | Nil |
| 8–10 hours | Capillary glucose | Nil |
| 24 hours | MRI – with contrast | 24 hours |
| Nil | MRI – no contrast | Nil |
| 24 hours | CT scan – with contrast | 24 hours |
| Nil | CT scan – no contrast | Nil |
| 24 hours | PET scan | 24 hours |
| Nil | X-Ray | Nil |
| Nil | Ultrasound | Nil |
| 1 whole day | Chemotherapy | 2 days |
| Ask Dr | Daily oral chemotherapy | Ask Dr |
| 2 whole days | Radiotherapy | 5 days |
| 24 hours | Surgery | Nil |
| 24 hours | Iron infusion | 24 hours |
Blood tests
High dose IVC may sometimes cause artefactual lab test results by interference in assays: decreased readings of cholesterol, triglycerides, uric acid; increased readings of sodium, potassium, calcium and creatinine (Kyle, et al., 2008).
Blood glucose test strips will show false elevation of readings with raised serum vitamin C because vitamin C can interfere with the chemical reaction on the glucose strip (Tang, et al., 2000).
NOTE: A laboratory blood serum glucose test is not affected (Jackson, et al., 2006).Blood testing precautions
Leave at least 24 hours between IVC infusions and renal function or creatinine blood tests to avoid false readings (Stephenson, et al., 2013).
People with diabetes
For insulin-dependent patients who rely on test-strip readings for their insulin dose, there is a risk of overdose causing hypoglycaemia.
Patients with diabetes should not rely on finger-prick (capillary) glucose tests until 8–10 hours after IVC treatment due to false elevation of readings when using test strips (Vasudevan, et al., 2014) – possibly even 12 hours after treatment.
PET scan
Blood glucose levels have a significant influence on PET scans as increased glucose levels can decrease 18F-FDG uptake in the brain and in tumours because of direct competition between binding sites and enzymes, which may lead to a false negative scan (Surasi, et al., 2014).
As IVC will cause a falsely elevated reading on glucometers therefore, it should be avoided for 24 hours prior to PET scan (Bahr, et al., 2014; Vasudevan, et al., 2014).
MRI/CT with contrast
Studies showed the contrast materials currently used – eg: gadolinium for MRI, iodine-based compounds for CT – are generally safe in patients with normal kidney function (dysfunction incidence approximately 2%) (Goldfarb, et al., 2009). In these patients, the contrast medium injected is almost entirely passed out of the body within 24 hours.
In rare cases, the contrast can cause kidney dysfunction like contrast-induced nephropathy (CIN), mostly in patients with impaired renal function and/or in those with diabetes. CIN generally occurs within 24 hrs of contrast administration, usually peaking on the third to fifth day, and returning to baseline within 10–14 days. (Andreucci, et al., 2014).
One study has shown a protective effect on the kidneys of oral vitamin C administered before and after contrast (Spargias, et al., 2004).
There were no reported nephropathy issues with IVC at the Clinic, and the Doctors' consensus was that stand down should be 24 hours before and after CT or MRI with contrast, but oral vitamin C could be continued.
Surgery
Before surgery
Some authors have shown positive effects of IV Vitamin C administered just before and during surgery, (less pain relief needed after palatal surgery, less evidence of myocardial injury from percutaneous coronary interventions) but only at lower doses, such as a few grams (Wang, et al., 2014; Ayatollahi, et al., 2016).
As IVC can affect blood test results, it is best to avoid IVC for 24 hours before surgery.
Anaesthesia
There are no clinical studies of IVC and anaesthesia. Animal studies suggest that vitamin C (given intramuscularly) before anaesthetic potentiates or enhances the effects of some anaesthetic agents.
Recommendations regarding IVC and anaesthetic are the same as those for surgery.
Animal references: Vitamin C can potentiate the effect of Ketamine in rabbits (Elsa, et al., 2005) and rats (Najafpour, et al., 2007).
After surgery
No stand down time is needed if the patient is able to attend.
Chemotherapy and radiotherapy
The results of recent clinical trials showed that combining IVC with chemotherapy or radio-chemotherapy appeared to be safe, well tolerated and could effectively decrease standard therapy associated side effects. Oral chemotherapy combined with IVC also seems safe, well tolerated and may increase survival time in some tumours (GBM). (Bael et al., 2008; Monti et al., 2012; Welsh et al., 2013; Kawada et al., 2014; Ma et al., 2014; Hoffer et al., 2015; Schoenfeld, et al., 2017; Zhao et al., 2018; Carr et al., 2018; Allen et al., 2019).
However, due to their small sample sizes and limited studies on radiotherapy, it is recommended patients avoid IVC one day before and two days after chemotherapy; avoid IVC two days before and five days after radiotherapy.
If oral chemotherapy is given every day, normal stand-down times do not apply. Some of the clinical trials used IVC in conjunction with oral chemotherapy (Bael et al., 2008; Schoenfeld, et al., 2017; Allen et al., 2019). IVC may be given on a case by case basis after discussion with the patient.
Iron infusion
Ferinject or other iron infusion. There are theoretical concerns that some of the circulating iron may be chelated by the vitamin C or that vitamin C and high concentrations of infused iron may undergo a Fenton type reaction causing tissue damage. Studies show mean plasma clearance range from 2.6–4.4 mL/min and terminal half-life from 7–12h. (Medsafe data sheet).
In the absence of published evidence of the safety of combining these treatments, it is advisable to leave 24 hours before and after iron infusion.
Additional resources
6.3.8. Possible Effects of IVC – Patient Guidance
The following is material the Clinic provided to patients:
Common effects
During and after an infusion of vitamin C, you may experience some common effects. Most are mild and may last up to a few hours.
- Dehydration – You may feel thirsty during and after a vitamin C infusion. Please drink plenty of fluids, unless you are on a restricted fluids regime. If so, please discuss with our doctors.
- Reduced blood sugar – A vitamin C infusion can reduce your blood glucose level. Having a meal before your treatment and snacking during treatment reduces your risk of this. Please tell the nurses if you notice yourself sweating or feeling dizzy, shaky, or nauseous. We may need to give you a glucose drink to help you feel better.
- Cramps, headaches, tingling, numbness – You may experience cramps, headaches, tingling or numbness during the infusion. Tell a nurse if you feel shaky. We may need to give you a calcium and magnesium drink to help you with this.
- Irritation or pain – If you notice any irritation or pain during your infusion, please tell a nurse immediately. Sometimes if you move around, the needle gets dislodged and the infusion fluid will cause pain. Do your best to keep your arm still throughout the infusion. Occasionally people will notice increased pain at the site of a recent injury or trauma during an IVC infusion.
- Tiredness – You may feel tired after your treatment. Rest and drink plenty of fluids. This effect usually goes away after the first few infusions.
- Blood tests – A vitamin C infusion can make some blood test results artificially high (eg: creatinine level). Do your blood test before treatment or more than 24 hours after the infusion.
- Finger-prick glucose testing – For people with diabetes, do not rely on finger-prick (capillary) glucose test results for 8–10 hours after your treatment because a vitamin C infusion can make the results artificially high. A laboratory blood serum glucose test is not affected, however.
Uncommon effects
There are also several possible effects which are not common, and require medical attention:
- Redness, pain, or swelling – If you develop any redness, pain, or swelling after treatment please contact the Clinic staff urgently. If it is outside business hours, see a doctor at A&E or your GP's after-hours service.
- Sore kidneys or blood in urine – The Clinic monitors your kidney function while you have IVC treatment because there is a reported rare risk of increased kidney stones. The Clinic has not seen this in its patients. If you do notice pain in your kidney region (flanks) or any blood in your urine, please contact the Clinic staff.
- Darkened urine or jaundice – Some people have a genetic deficiency that means higher doses of vitamin C are inadvisable. The Clinic always test your G6PD enzyme level before giving more than 25g/30g of vitamin C. If you notice darkened urine or jaundice of your skin or eyes, please contact the Clinic urgently. If it is outside of business hours, see a doctor at A&E or your GP's after-hours service.
6.4. Delivering treatment
6.4.1. Equipment needed
Prepared IV bag with additives and primed IV giving set, tourniquet, selected butterfly or cannula needle, alcohol swabs, gauze square, prepared tape and/or opsite, felt tip pen.
6.4.2. Storing supplies
Store and dispose of injectables and other supplies as directed on their packaging and datasheets.
Ascorbic Acid or Sodium Ascorbate for injection should be kept refrigerated.
6.4.3. Preparing intravenous solutions
Each clinic has its own way of managing the preparation of IV bags. These are some helpful tips the Clinic picked up during its practice:
- Follow the specified storage temperatures and disposal periods for injectables to preserve their efficacy. After Sodium Ascorbate or ascorbic acid is mixed with water, it should be used within 6 hours or disposed of.
- Keep the preparation environment clean, and also use alcohol swabs for the injection ports on IV bags and the tops of any opened vials. Use asceptic technique throughout.
- Preparing bags requires focus so discourage others from interrupting.
Using Ascorbic Acid
The two forms of injectable vitamin C used are Ascorbic Acid or Sodium Ascorbate.
| Ascorbic Acid | Sodium Ascorbate | |
|---|---|---|
| Concentration | 25g / 50mL | 30g / 100mL |
| Vial volume | 50mL | 100mL |
Carrier solutions
The carrier solution for administration can be 0.9% saline, sterile water for injection, or glucose 5%.
The dosage of Ascorbic Acid/Sodium Ascorbate may affect which carrier is prescribed. For more details, see the Injectable Preparations section above.
Bag volumes
The following are the Clinics recommendations for preparing solutions. Bag draining may be required.
| Ascorbic Acid (50 mL vial) | |
|---|---|
| Ascorbic Acid dose | Bag preparation |
| < 50g | Use a standard 250 mL bag of carrier solution. |
| 50–100g | Use a 500 mL bag of sterile water for injection. |
| > 100g | Drain 200 mL or more from a 1,000 mL bag of sterile water for injection. |
| Sodium Ascorbate (100 mL vial) | |
|---|---|
| Sodium Ascorbate dose | Bag preparation |
| < 35g | Use a standard 250 mL bag of carrier solution. |
| 35–49g | Drain 50 mL from a standard 250 mL bag. |
| 50–90g | Drain 100 mL from a 500 mL bag of sterile water for injection. |
| > 90g | Drain 200 mL or more from a 1000 mL bag of sterile water for injection. |
Calculate drip rate
Infusion rate for intravenous vitamin C must be no faster than 1 gram per minute.
- Volume = total volume of fluid in the IV bag, including all additives.
- Drip factor is stipulated on the IV giving set.
- It is recommended that the calculated drip rate is written on the bag directly.
Calculate: minimum* time (minutes) = IVC dose (grams),
(volume (mL) × drip factor (drops/mL)) ÷ time (minutes) = drips per minute
* Adjust upwards depending on the patient. Use a lower rate for first treatment/s and monitor tolerance.6.4.4. Administering infusions
Pre-treatment patient check
As part of our standard clinical care, the Clinic's nurses administered a brief check before each treatment session. If there are any concerns about a patient's readiness for treatment, then an urgent doctor consultation is arranged. Questions, along the lines of the following can be asked:
- Q1 – How were you after your last IV treatment?
-
Q2 – Since your last visit here, have you had any change in your condition or treatment schedule?
(prompt further if needed)…- seen a doctor or specialist,
- been to hospital,
- had or planning any scans or other treatment like chemo or radiotherapy,
- changed the medication or supplements you take,
- had any abnormal events (like vomiting or fever)?
- Q3 – Have you eaten?
Infusing
These are The Clinic's guidelines for administering an infusion:
- Check patient name, date of birth and vitamin C dose on IV bag with patient and against treatment sheet.
- Mark bag with start time, halfway, and expected finish time.
- Do not connect the IV if you feel there is a problem. If you are concerned about the patient showing unusual pallor, breathlessness, pulse, BP, or agitation, please alert doctor.
- Wash hands and insert cannula or 23-gauge butterfly needle, secure and connect IV.
- Set IV drip rate as calculated. Monitor every 5 minutes or so, as drip rate can alter very easily.
- Keep an eye on patient's IV site for any signs of tissuing (infiltration/extravasation). Instruct patient to inform nurse when they have pain at site or feel unwell in any way.
- Provide oral fluids (water, tea, juice) as required throughout the IV process, as IVC can cause mild dehydration and thirst. At doses above 30 grams observe for signs of hypoglycaemia.
- When an IV is removed, instruct patient to press firmly on the site for three minutes (or longer if the patient is taking anticoagulants since bleeding time will be prolonged).
6.4.5. Managing infusion problems
Emergencies
If a patient's condition requires urgent medical attention, follow your emergency procedures. Examples are: anaphylaxis, choking, collapse, hypocalcaemia, hypoglycaemia.
Symptoms and problems
| SYMPTOMS | EXPLANATION |
|---|---|
| Pain Discomfort |
If patient complains of pain, act immediately. The patient may also feel faint because of the pain. ACTION
|
| Swelling Discomfort Burning Pain Tightness |
Infiltration/Extravasation IV fluid may leak into the surrounding tissue. It is commonly caused by needle dislodgement, patient movement or improper placement of the needle. The risk increases in older patients as their veins are thin and fragile. ACTION
NOTE: it is possible for an IV to continue to run into the tissue and for the patient to feel no pain. |
| Nausea Trembling/shakiness Blurry vision Headache Decreased co-ordination Impaired judgement |
This may be due to hypoglycaemic effect of IVC. Once resolved, remind patient to eat before IV treatment, and to have a snack during the IV treatment. ACTION
|
| Tingling mouth or extremities Numbness Muscle spasm/cramp Diplopia Stridor Tetany Convulsions Positive Trousseaux and Chvostek signs |
This may be due to hypocalcaemic effect of IVC. ACTION
|
| Lightheaded Faint |
ACTION
|
| Arm numbness | May be caused by arm position. ACTION
|
Preventing contamination
If the IV needle has to be re-sited, protect the IV giving set from contamination. Once the needle is removed, disconnect it from the giving set and attach a blue luer lock to the exposed end of the giving set.
Discard the IV needle, following your clinic's Infection Control procedure.
Preventing haematoma
After removal of IV needle, ensure the patient knows to apply pressure to the site for a minimum of three minutes.
If patient is on blood thinning medication encourage them to hold the site for longer; up to 5 minutes. Consider using an IV pressure pad for these patients.
Documentation
Any problems that have occurred during the patient's IV treatment should be recorded in your patient management system.
Additional resources
6.5. Summary of Module 6
- Ascorbic acid for infusion (ASCOR L 500®) has MedSafe approval in New Zealand. Sodium Ascorbate Solution (Biological Therapies) is available in Australia. These are typically used for immune support and to support people with cancer.
- Indications for vitamin C infusions include scurvy, vitamin C insufficiency. It is also given for viral/bacterial infections, cancer, autoimmune disorders, mercury amalgam removals, post-operative wellbeing.
- Contra-indications include acute renal failure, previous allergic reaction to vitamin C administration. Precautions include G6PD, impaired renal function, pre-existing renal stones, congestive heart failure, severe thrombocytopenia. There is as yet no safety data for pregnancy or breastfeeding.
- Ascorbic acid can be diluted into water for injection, or saline 0.9% or glucose 5% for doses < 50 g. Doses of 15–25 g (~200 mg/kg body weight) are typically used for immune support and doses of 25–50 g (and up to 1 g/kg body weight) are typically used to support people with cancer. Infusion rate for IVC must be no faster than 1 gram per minute.
- Infusions are typically administered 1–2 times per week. Written informed consent should be sought before beginning IVC treatment and when assessing the risk level of IVC treatment for a patient, both clinical and ethical factors should be considered.
- G6PD deficiency may result in haemolysis with high dose IVC. The patient's G6PD level should be tested before giving more than 25g of Ascorbic Acid (normal range for G6PD is 7–20 U/g Hb).
- Poor initial or unnoticed subsequent deterioration in patient renal function may affect ability to handle IVC treatment. The renal function of patients should be monitored throughout a course of treatment and any changes managed.
- IVC can interact with some other medical treatments and investigations. Refer to the stand-down times for the desirable intervals between IVC treatment and other forms of medical treatment or investigation, e.g. some blood tests, some MRI/CT scans, PET scans, some chemotherapy, radiotherapy.
- Daily oral vitamin C is recommended to be taken in conjunction with the IVC protocols, including on the days of intravenous treatments. Other oral supplements, e.g. daily lipoic acid, are also recommended.
- Possible effects of IVC infusions include dehydration, hypoglycaemia, cramps/headaches/tingling/numbness, irritation/pain, tiredness. Uncommon effects include redness/pain/swelling, sore kidneys/blood in urine, darkened urine/jaundice.
6.6. Activity: Self-reflection and feedback
Once you have completed all the Modules of interest to you, please complete the self-reflection and feedback form online or email to anitra.carr@otago.ac.nz. For members of the RNZCGP, please include your MCNZ number and the modules completed so that your CPD credits can be uploaded to the RNZCGP website. A certificate of completion can be emailed to you if required.
- Self-reflection and feedback form (online)
- Self-reflection and feedback form (DOCX)
© Anitra Carr, 2022. You may not copy, modify, distribute, display, transmit, perform, publish or sell any of the copyrightable material on this website. You may hyperlink to this website but must include the following statement: “This link leads to the website 'Oral and intravenous vitamin C use in health care' provided by the Nutrition in Medicine Research Group at University of Otago, Christchurch.”
