3.1. Overview of Module 3
In this module you will learn about the various roles of vitamin C in the prevention and treatment of infection and sepsis. The infections covered are: upper respiratory tract infections (e.g. common cold), lower respiratory tract infections (e.g. pneumonia), and sepsis/septic shock, which are life-threatening complications of severe infections. The use of both oral and intravenous vitamin C in relation to these conditions will be discussed.
Following completion of this module the participant will be able to:
- Discuss the evidence-based role of vitamin C in infection prevention and therapy to colleagues and patients (Communication, Scholarship, Professionalism)
- Explain the rationale for oral versus intravenous vitamin C use in infection and sepsis to colleagues and patients (Communication, Scholarship, Professionalism)
- Describe how vitamin C works in infection and sepsis to colleagues and patients (Communication, Scholarship, Professionalism)
- Assess the potential usefulness of vitamin C administration to patients with infection or sepsis in their healthcare practice (Management)
- Apply knowledge on vitamin C administration to patients with infection or sepsis in their healthcare practice (Clinical expertise)
Length of activity: 75 min (25 min reading + 50 min activity)
RNZCGP Endorsed: 1.25 CME credits
CICM Accredited: 1.25 points
3.2.Connection between vitamin C status and infection
Research from the Global Burden of Disease initiative indicates that respiratory infections are a leading cause of morbidity and mortality globally, particularly in the very young and the elderly. Severe respiratory infections, such as pneumonia, are one of the most common complications and causes of mortality in people with severe vitamin C deficiency (scurvy). This suggests an important link between vitamin C deficiency and severe infection. In confirmation of this premise, epidemiological evidence from the large EPIC-Norfolk study has indicated that people with the highest vitamin C status had lower risk (HR 0.70; 95% CI 0.6, 0.8) and mortality (HR 0.61; 95% CI 0.4, 1.0) from pneumonia (see figures below).
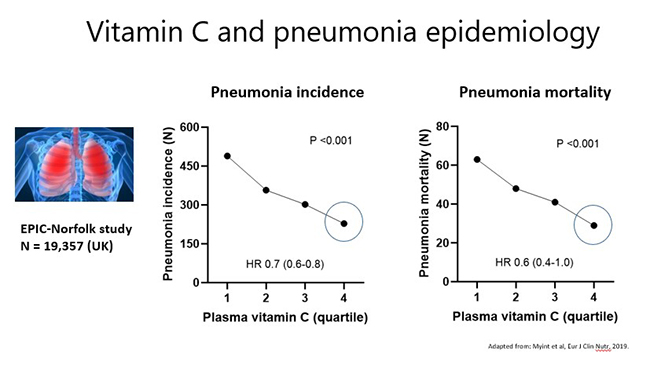
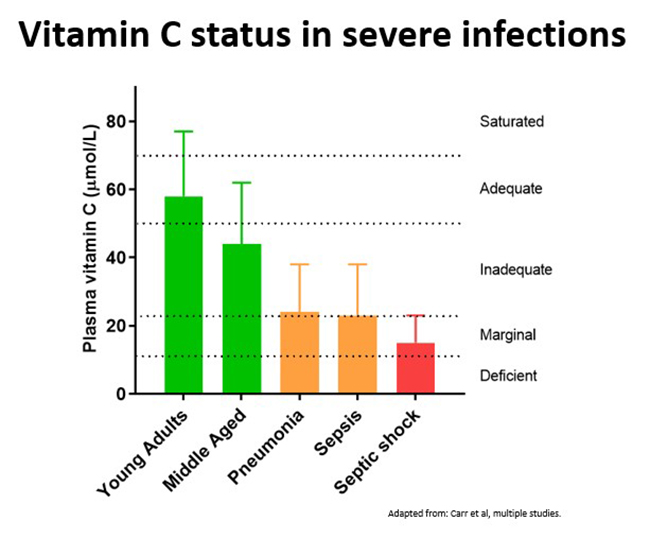
Conversely, severe infections can significantly deplete vitamin C levels due to enhanced utilization of the vitamin during the infectious process. The extent of depletion depends on the severity of the condition (see figure below), with septic patients in ICU having by far the lowest levels of vitamin C (discussed in further detail below).
Further reading:
- Vitamin C in pneumonia and sepsis. Carr, A.C. In: Vitamin C: New Biochemical and Functional Insights. CRC Press/Taylor & Francis. 2020. Chapter 7.
This book chapter summarises the studies that have investigated vitamin C status in pneumonia (Table 7.1) and sepsis (Table 7.3).
3.2.1.Vitamin C status in the common cold
The common cold is a relatively mild viral upper respiratory tract infection which generally doesn't last longer than a week. Of note, coronaviruses are one of the many types of viruses that can cause the common cold (SARS-CoV-2 infection will be specifically covered in a later section).
One study found that leukocyte vitamin C status was decreased by 50% during the early stages of common cold symptoms (see figure below). Because of the important roles that vitamin C likely plays in leukocyte function, this decrease in vitamin C concentrations may impact negatively on leukocyte function (discussed in more detail below). In support of this premise, case reports of recurrent infection treated with vitamin C have shown improved leukocyte functions as well as resolution of infection in many cases.
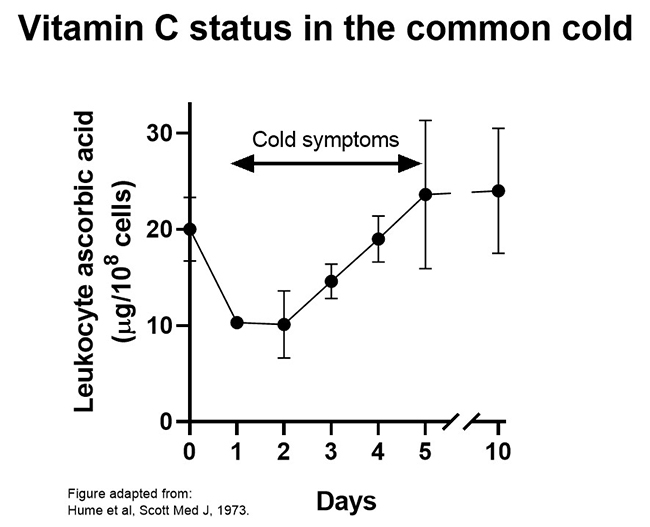
3.2.2.Vitamin C status in pneumonia
Pneumonia is a severe lower respiratory tract infection that can be caused by bacterial, fungal and viral pathogens. Lower respiratory tract infections are the leading cause of morbidity and mortality for communicable disease worldwide.
Vitamin C levels in patients with pneumonia are approximately 50% of healthy controls, although this depends on the severity of the condition, with those in ICU and those who died having the lowest levels (see figure below). Up to 40% of patients with pneumonia exhibited outright vitamin C deficiency (i.e., plasma vitamin C levels <11 μmol/L), and levels can remain low for at least 4 weeks. Of note, significantly elevated levels of oxidative stress biomarkers (protein carbonyls) were detected in the circulation of patients with pneumonia.
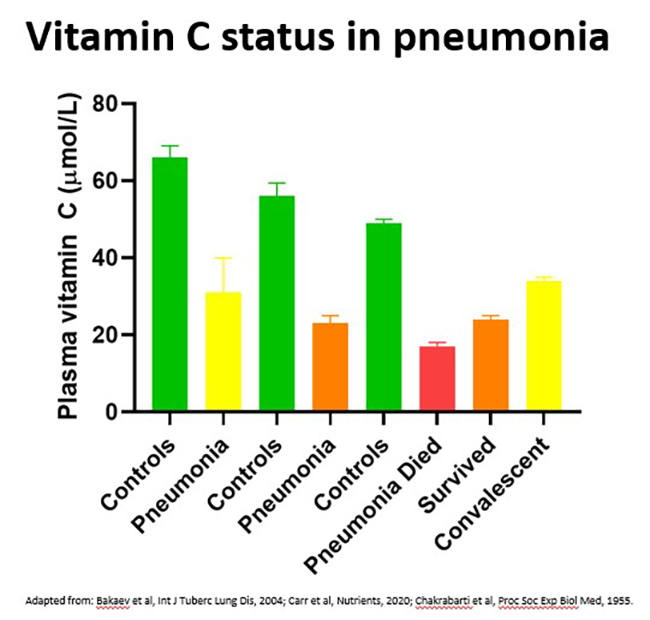
Further reading:
- Patients with community acquired pneumonia exhibit depleted vitamin C status and elevated oxidative stress. Carr et al. Nutrients, 2020.
This research paper highlighted the depleted vitamin C status of hospitalised patients with pneumonia, with the severity of the depletion relating to the severity of the disease.
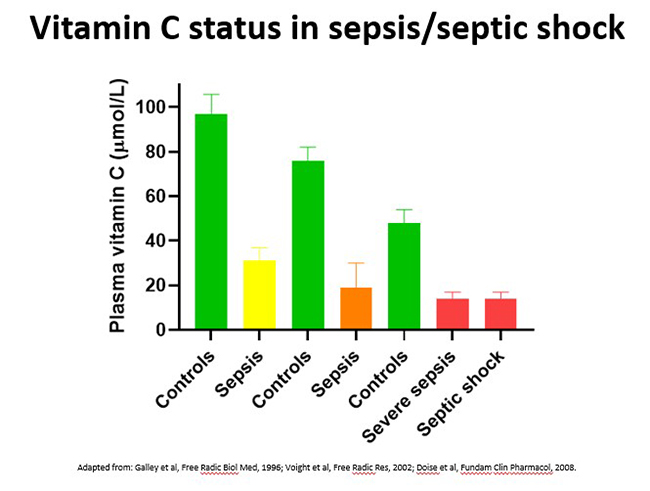
3.2.3.Vitamin C status in sepsis/septic shock
Sepsis is a life-threatening condition comprising organ dysfunction due to a dysregulated host response to infection. Sepsis is a growing global health issue, with an estimated 49 million cases worldwide and is responsible for 20% of annual global deaths. Septic shock is a more severe clinical presentation characterised by profound circulatory, cellular, and metabolic abnormalities and is associated with mortality rates greater than 40%.
Critically ill patients with sepsis/septic shock have the lowest vitamin C levels of all, i.e. approximately one third that of healthy controls (see figure below - up to 40% exhibit deficiency and nearly 90% have hypovitaminosis C). Lower vitamin C status in these patients was associated with increased inflammation (C-reactive protein levels), increased severity of the illness (days in the ICU), and multiple organ failure.
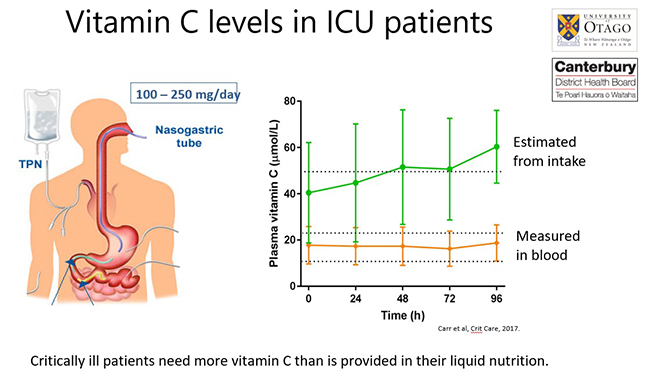
The lower vitamin C status of ICU patients occurs despite that fact that they are generally receiving recommended enteral/parenteral intakes of the vitamin (see figure below). This indicates that these patients have significantly higher requirements for vitamin C than is provided by their liquid nutrition.
Further reading:
- Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Carr et al. Crit Care, 2017.
This research paper highlighted the depleted levels of vitamin C in critically ill patients (including those with sepsis), despite receiving recommended intakes.
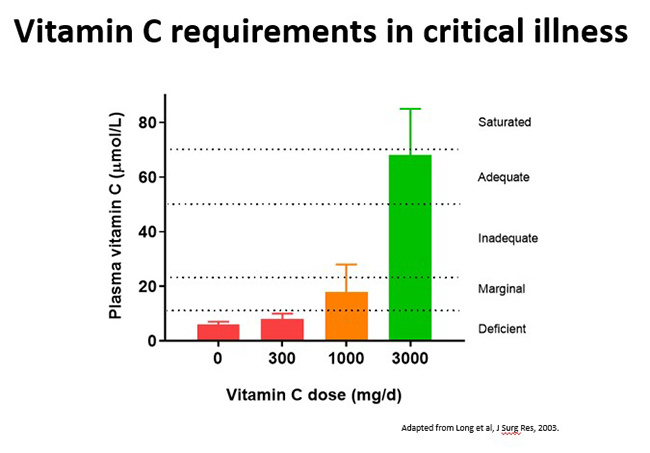
3.3.Vitamin C pharmacokinetics in critically ill patients
Critically ill patients, including those with sepsis, have enhanced requirements for vitamin C. Recommended intakes that would normally saturate the plasma of healthy people (i.e. 200–300 mg/day) are insufficient. Instead these patients require parenteral administration of 10 times this amount (i.e. 2–3 g/day) to replete their plasma vitamin C status (i.e., ∼70 μmol/L) (see figure below).
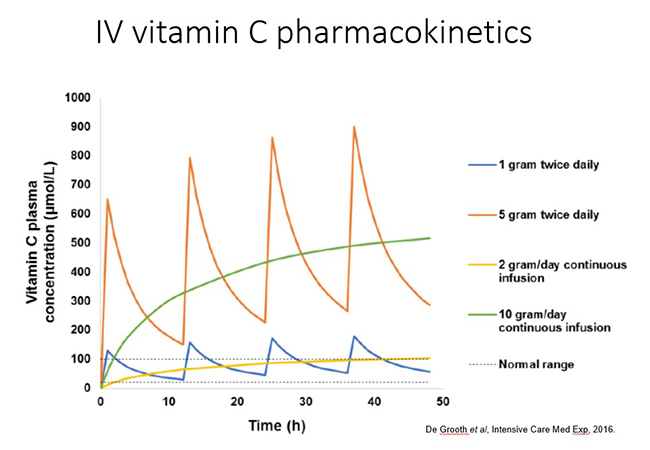
In trials of critically ill septic patients, intravenous vitamin C is generally administered in 2-4 bolus doses over the day. This produces transient peaks in plasma followed by rapid renal clearance of excess vitamin (see figure below). Vitamin C can also be administered as a continuous infusion in the ICU. Pharmacokinetic data indicated that administration of 2 g/day of vitamin C to critically ill patients, as either bolus or continuous infusions, resulted in plasma concentrations in the normal range. However, it should be noted that hypovitaminosis C occurred in some patients following cessation of the intervention, suggesting sustained therapy may be required to prevent this from occurring. It is not yet known which administration method results in the most efficacious outcomes.
3.4.Oral and intravenous vitamin C use in infection and sepsis
Both oral and intravenous vitamin C have been used prophylactically and therapeutically for infections and sepsis. The vitamin C dose and route of administration depends upon the severity of the condition, with oral doses of ≥200 mg/day being used to prevent respiratory infections (e.g. common colds and pneumonia) and restore vitamin C status, to gram intravenous doses being used therapeutically in critically ill patients with sepsis.
3.4.1. Vitamin C use in the common cold
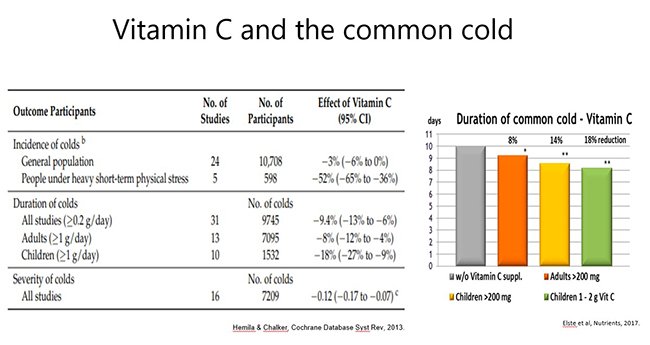
Prophylactic administration: Meta-analysis of trials assessing vitamin C for the prevention and treatment of the common cold has indicated that members of the general population who regularly consumed ≥200 mg/day vitamin C (~30 trials) did not necessarily have a decreased risk of getting common colds, however, the severity and duration of cold symptoms was decreased (by up to 18% in children; see figure below).
Of note, adults who were under enhanced physical stress and/or exposed to cold temperatures (5 trials) had a 50% decreased risk of getting common colds with prophylactic supplementation. Both physical and psychological stress are known to impact negatively on the immune system. Vitamin C is believed to contribute to the body's response to stress, particularly during severe infections.
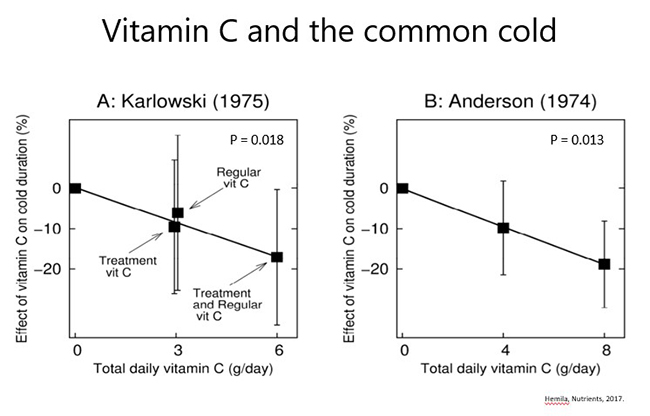
Therapeutic administration: of oral gram doses of vitamin C (e.g. 1-8 g/day) following onset of cold symptoms (7 trials) exhibited some benefit on severity and duration of symptoms in several studies; there appeared to be a dose-dependent effect in a couple of trials (see figures below). Of note the Anderson trial (shown below) administered vitamin C only on the first day of the cold, so it is possible that greater effects might be seen if it was given more regularly and for a longer period. Consumption of divided doses throughout the day may also be of more benefit than a bolus daily dose; this remains to be tested.
Further reading:
- Vitamin C for preventing and treating the common cold. Hemilä and Chalker. Cochrane Database Syst Rev, 2013.
This meta-analysis of common cold trials comprised analysis of 34 prophylactic risk trials, 31 prophylactic duration trials and 7 therapeutic trials. The findings are summarised above.
3.4.2.Vitamin C use in pneumonia
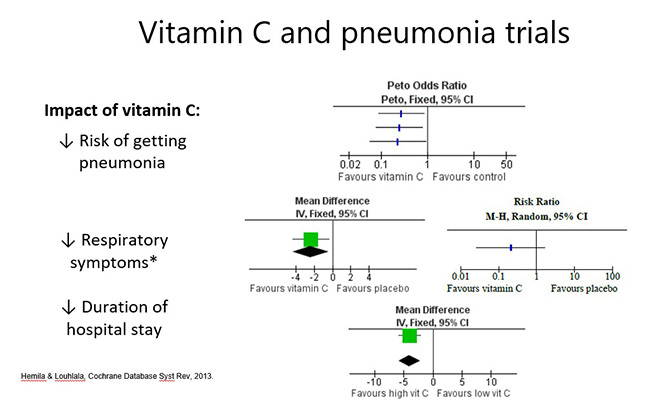
Prophylactic administration: Cochrane meta-analysis of trials assessing vitamin C for the prevention and treatment of pneumonia has indicated that prophylactic consumption of ≥200 mg/day vitamin C (three trials) deceased the risk of getting pneumonia (by ≥80%; see further reading below). One of these trials was in adults with influenza A, which indicates that administration of vitamin C to patients with respiratory infection may prevent it from developing into the more severe condition of pneumonia.
Therapeutic administration: To date, only a couple of trials have tested therapeutic administration in adults; one showed decreased severity of symptoms, particularly in the most severely ill (and a trend towards decreased mortality); and the other showed a dose-dependent decrease in hospital length of stay (see figure below). Decreasing the severity of pneumonia may decrease its progression to the more severe conditions of sepsis and septic shock.
A more recent Cochrane meta-analysis included trials carried out in children (see further reading below). A couple of therapeutic trials carried out in ≤5 year old children in Pakistan showed shorter duration of hospital stay and shorter duration to respiratory rate improvement. Note that although children from high-income countries tend to have higher vitamin C status than adults, children from low-middle-income countries are likely to be low in vitamin C even prior to acquiring pneumonia.
Further reading:
- Vitamin C for preventing and treating pneumonia. Hemilä and Louhiala. Cochrane Database Syst Rev, 2013.
- Vitamin C supplementation for prevention and treatment of pneumonia. Padhani et al. Cochrane Database Syst Rev, 2021.
These meta-analyses assessed 2-3 prophylactic trials and 2-3 therapeutic trials (discussed above). Note that of these authors, Hemilä is the most experienced with regard to vitamin C and infection.
3.4.3. Vitamin C use in sepsis/septic shock
In 2014 Dr Fowler and colleagues published a phase I trial investigating intravenous vitamin C as monotherapy in patients with sepsis. In this study, 24 patients with severe sepsis were treated with 0, 50, or 200 mg/kg body weight intravenous vitamin C per day, which provided a dose-dependent decrease in systemic organ failure and decreased pro-inflammatory (C-reactive protein and procalcitonin) and tissue damage (thrombomodulin) biomarkers. Although the study was not powered to detect a decrease in mortality, fewer participants died in the treatment arms.
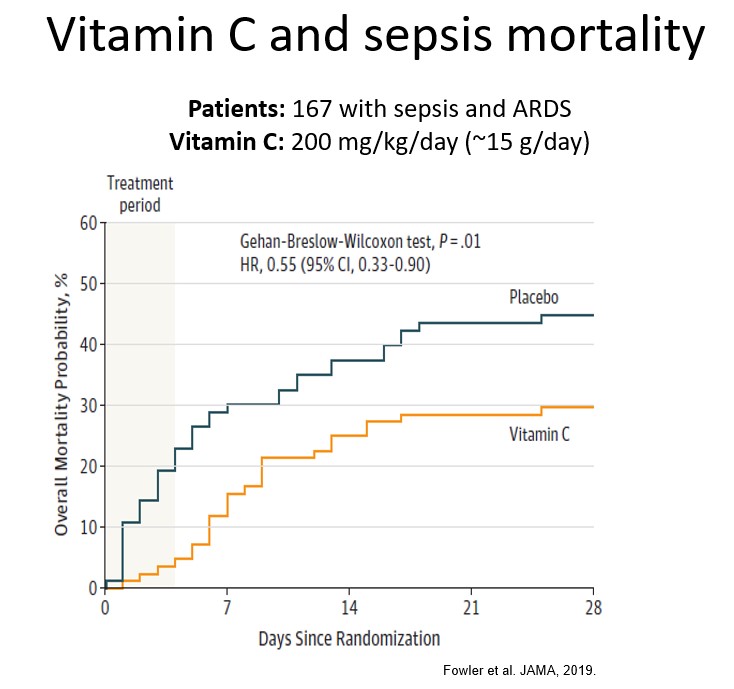
This trial was subsequently followed up by a multicenter double-blind randomised placebo controlled trial (the CITRIS-ALI trial) in 167 septic patients with acute respiratory distress syndrome (ARDS) administered 200 mg/kg/day vitamin C or placebo (published in well-respected medical journal JAMA). Although the trial did not detect any changes in the biomarkers measured in the above phase I trial, it did show improvements in several clinically relevant outcomes, including decreased mortality (HR 0.55 [95% CI 0.33-0.90], see figure below) and increased ICU and hospital-free days (see further reading below). Additional statistical analysis indicated that vitamin C intervention decreased mortality by 81% (95% CI 45-94) during the 4-day intervention period. This dramatic difference was lost in the period following intervention indicating that longer intervention may be required, which has been supported by other research indicating better outcomes with >5 days intervention.
Following publication (in 2017) of the well publicised Marik retrospective study, which showed a dramatic survival advantage in 47 septic patients who received a cocktail of IV vitamin C (6 g/day) in combination with hydrocortisone and thiamine (now known as 'HAT' therapy), many trials have been carried out investigating this combination. However, due to the combinatorial nature of the therapy, it is not certain which of the ingredients is having the effect. One trial (the VITAMINS trial) examined this further and showed that IV vitamin C administered with hydrocortisone had no additional survival advantage over hydrocortisone administered alone. Unfortunately, this trial did not include a third, vitamin C only, arm so the efficacy of vitamin C relative to hydrocortisone is still unknown.
A number of small IV vitamin C and sepsis trials have been published over the last few years, including one carried out at Christchurch Hospital, NZ. These have shown variable effects on outcomes, including potentially shortening ICU length of stay, however, most of these studies were not appropriately powered to detect clinical outcomes. Of note, better outcomes appear to be observed in studies carried out in low-middle income countries where the participants are likely to be already chronically vitamin C insufficient prior to the acute vitamin C insufficiency induced by severe infection.
As of early 2022, over 20 meta-analyses (and 2 component network meta-analyses) have been published around vitamin C (and/or HAT) intervention in critically ill and septic patients. However, there have been too few good quality (properly powered) RCTs specifically investigating IV vitamin C and sepsis to form any meaningful conclusions as yet. Of note, the large LOVIT trial, comprising 872 septic patients recruited in three countries, has recently been published (2022; NEJM). In contrast to the CITRIS-ALI trial, they did not find any beneficial effect of vitamin C on mortality. Subgroup analysis did, however, indicate better outcomes in the small group of patients who had SARS-CoV2 infection.
3.4.4. Activity: sepsis audiovisual
Timing: approx. 60 min.
Watch this presentation by Prof Berry Fowler on “Intravenous vitamin C: pathway to a new therapy to save lives” (from the Vitamin C Symposium: from bench to bedside).
3.4.5. Optional activity: sepsis podcast
Timing: approx. 45 min.
Listen to this podcast by Dr Alpha 'Berry' Fowler on Vitamin C in Sepsis and ARDS Treatment.
Further reading
- Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. Fowler et al. JAMA, 2019.
This RCT showed a survival advantage to patients with ARDS which received IV vitamin C, as well as more ICU and hospital-free days. - Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. François Lamontagne et al. NEJM, 2022.
This RCT did not show a survival advantage for short-term IV vitamin C in septic patients, but did indicated better outcomes in subgroup of patients with SARS-CoV2.
3.5.Oral and intravenous vitamin C use in COVID-19
Since the novel coronavirus (SARS-CoV-2) and its associated disease (COVID-19) were declared a global pandemic in early 2020, there has been a worldwide effort to establish therapies to prevent and treat the respiratory infection and more severe disease. In the absence of specific comorbidities that increase the risk of developing severe COVID-19, infection with SARS-CoV-2 can be relatively mild and even asymptomatic in some cases. It is interesting to note that there is significant overlap in the risk factors for COVID-19 and the risk factors for vitamin C deficiency.
Pneumonia and sepsis are major complications of severe COVID-19, therefore, based on the findings around vitamin C use in these conditions, it is possible that intervention may show some efficacy towards COVID-19. In fact, the World Health Organization (in their 'Coordinated Global Research Roadmap for the 2019 Novel Coronavirus' document) highlighted vitamin C as an adjunctive intervention with biological plausibility to improve the outcome of critical COVID-19 patients.
3.5.1.Vitamin C status in COVID-19
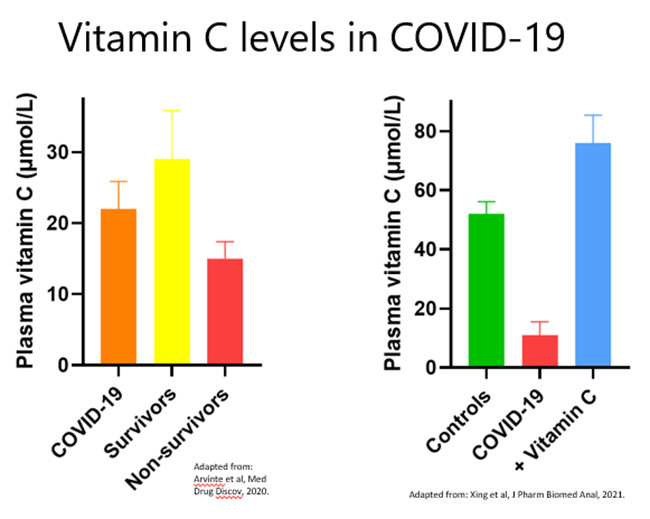
Of the studies published to date, the vitamin C status of people with severe COVID-19 is very low, i.e. comparable to other critically ill people with sepsis and ARDS (see figure below and Table 1 in further reading below. Also see the Vitamin C and COVID-19 Research Resource link below for links to the relevant papers).
3.5.2. Vitamin C use in COVID-19
Prophylactic administration: To date, no prospective prophylactic trials have been carried out. One case-control study has attempted to estimate the effect of regular vitamin C supplementation on the incidence of SARS-CoV-2 infection. Cases and controls were health-care workers who tested positive and negative, respectively, for SARS-CoV-2 infection. Of the 372 participants, 67 participants took vitamin C supplements (500 mg) once or twice daily. There was, however, no significant association with SARSCoV-2 infection compared with the control group.
The outcomes from this study correlate with previous research indicating that prophylactic vitamin C did not decrease the risk of the common cold in the general population, except in specific subgroups who experienced enhanced physical stress. However, prophylactic vitamin C has been shown to decrease the risk of more severe respiratory conditions such as pneumonia. As such, regular vitamin C supplementation may prevent mild SARS-CoV-2 infection from progressing to the more severe conditions of pneumonia and sepsis that are observed in critical COVID-19, thus potentially decreasing the need for hospitalisation and intensive care.
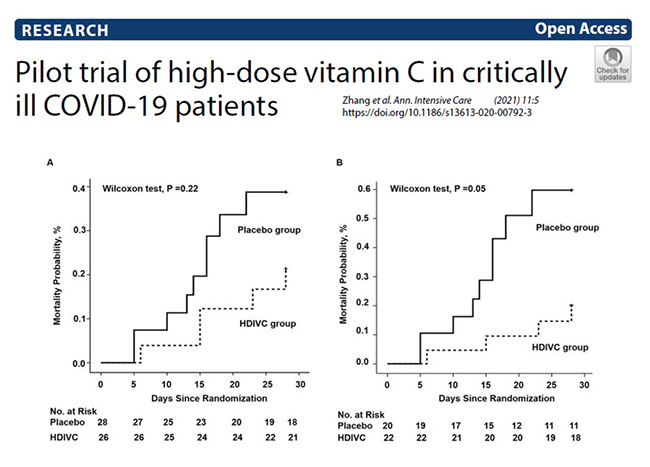
Therapeutic administration: There are numerous clinical trials currently underway to test the efficacy of oral and intravenous vitamin C for COVID-19 (see clinicaltrials.gov link below). The first placebo-controlled RCT to be published (out of Wuhan) administered a relatively high intravenous vitamin C dose of 24 g/day (12 g every 12 hours) for 7 days versus placebo. This trial was terminated early due to lack of patients. Nevertheless, they recruited 54 patients and saw an increase in oxygenation parameters (increased PaO2/FiO2) and a decrease in ICU and hospital mortality in patients with SOFA scores ≥3 (p = 0.03; see figure below for 28-day mortality). Other trials carried out in low-middle income countries have shown increased rates of recovery and fewer days spent in hospital.
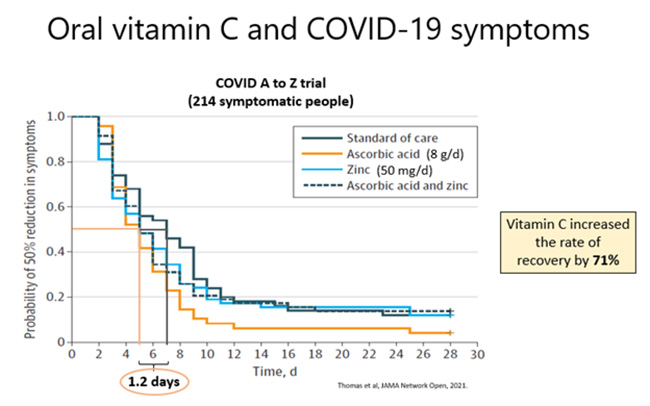
To date, one published trial has investigated the effects of oral vitamin C supplementation on SARS-CoV-2 symptoms. This trial was underpowered as it was halted early, but nevertheless showed a 1.2-day trend towards decreased duration to reach 50% reduction in symptoms in the participants who received supplemental vitamin C (8 g/day). Furthermore, independent statistical analysis of the results showed a significant 70% increase in the rate of recovery in the vitamin C group compared to standard care. Thus, both oral and IV vitamin C trials are showing indications of benefit in patients with SARS-CoV-2 infection and critical COVID-19. Larger trials (e.g. LOVIT-COVID and REMAP-CAP) are currently underway and will hopefully provide more definitive findings.
Additional resources:
- Vitamin C and COVID-19 Research Resource - links to the latest research
- Vitamin C for COVID-19: real-time meta analysis - studies as they are published
- clinicaltrials.gov - for information on registered vitamin C and COVID-19 trials
Further reading:
- Vitamin C Intervention for Critical COVID-19: A Pragmatic Review of the Current Level of Evidence. Holford et al. Life (Basel), 2021.
This review summarises the trials which have investigated vitamin C status and intervention in patients with SARS-CoV-2 infection and COVID-19.
3.6.How much vitamin C to use for infection/sepsis/COVID?
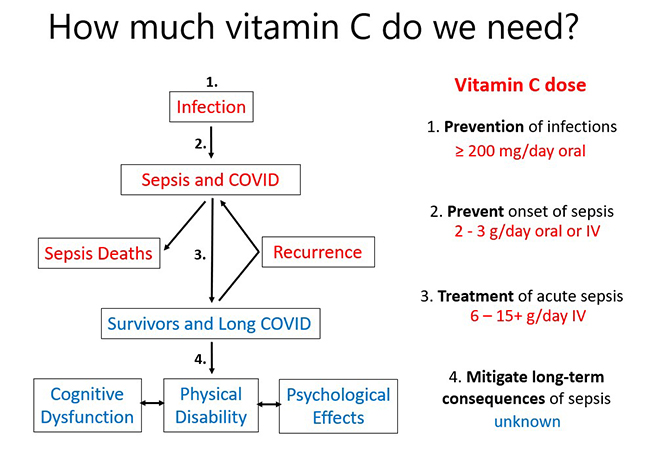
To summarise, vitamin C requirements increase as the severity of the illness increases (see figure below).
Meta-analysis has indicated that intakes of at least 200 mg/d vitamin C will decrease the risk of acquiring an infection, particularly if the person is under enhanced stress.
If a person does come down with an infection, their requirements for vitamin C increase significantly, and increasing their vitamin C dose to 2-3 g/day may prevent it from developing into a more serious condition, such as pneumonia, or sepsis.
If the person does develop sepsis or severe COVID-19, clinical studies indicate that administration of 6-15+ g/day of intravenous vitamin C to patients may be able to decrease symptoms and improve outcomes including potentially decreasing mortality.
Survivors of sepsis can have severe long-term health conditions including cognitive dysfunction, physical disability and psychological effects such as anxiety and depression. This is also observed in some people following COVID-19 and is termed 'long COVID'. The effect of vitamin C on long-term quality of life outcomes in survivors has not yet been assessed, although this is being monitored in current large trials. However, it is likely that short term IV vitamin C administration (for 4 days) may not have long-term benefit due to the ongoing needs of the patients. Therefore, in order to maximise long-term benefit, patients should be encouraged to go onto oral vitamin C supplementation once discharged from hospital.
3.7.Vitamin C mechanisms of action in infection/sepsis/COVID-19
3.7.1.Anti-inflammatory and antioxidant functions
Patients with pneumonia, sepsis and COVID-19 typically have elevated markers of inflammation (e.g. C-reactive protein, procalcitonin and interleukins – the latter is sometimes referred to as a 'cytokine storm'). Several small trials have indicated decreased inflammatory biomarker levels (e.g. CRP, PCT, IL-6) following intervention with intravenous vitamin C, although the CITRIS-ALI trial did not show any effect of intervention on CRP levels in septic patients with ARDS.
Pro-inflammatory states are also typically associated with enhanced oxidative stress as evidenced by elevated levels of oxidative stress biomarkers (e.g. oxidised lipid and protein molecules). Few studies have investigated the effects of vitamin C intervention on markers of oxidative stress in septic patients. One small trial has shown that short-term (4 day) administration of intravenous vitamin C to patients with severe pneumonia was not able to attenuate the elevated markers of protein oxidation (see further reading below).
Further reading
- Circulating protein carbonyls are specifically elevated in critically ill patients with pneumonia relative to other sources of sepsis. Spencer et al. Free Radic Biol Med, 2022.
This research paper showed significantly elevated protein oxidation markers in septic patients with pneumonia, however, administration of IV vitamin C was not able to attenuate these elevated levels.
3.7.2.Support of immune system
Vitamin C has numerous roles in that help support optimal function of both the innate and adaptive immune system (see further reading below). For example, the vitamin supports epithelial cell barriers through enhancing collagen synthesis and stabilization, protecting against ROS-induced damage, enhancing keratinocyte differentiation and lipid synthesis, and enhancing fibroblast proliferation and migration.
The vitamin also supports various phagocyte (neutrophil) functions through acting as an antioxidant/electron donor, enhancing motility/chemotaxis, enhancing phagocytosis, ROS generation and microbial killing, facilitating apoptosis and clearance whilst decreasing necrosis/NETosis (see figure below). Case series have shown improved neutrophil function and resolution of recurrent infections following vitamin C supplementation.
Vitamin C also supports lymphocyte differentiation and proliferation. These functions are likely due to its effects on epigenetic regulation. It may also help modulate antibody, cytokine and histamine levels.
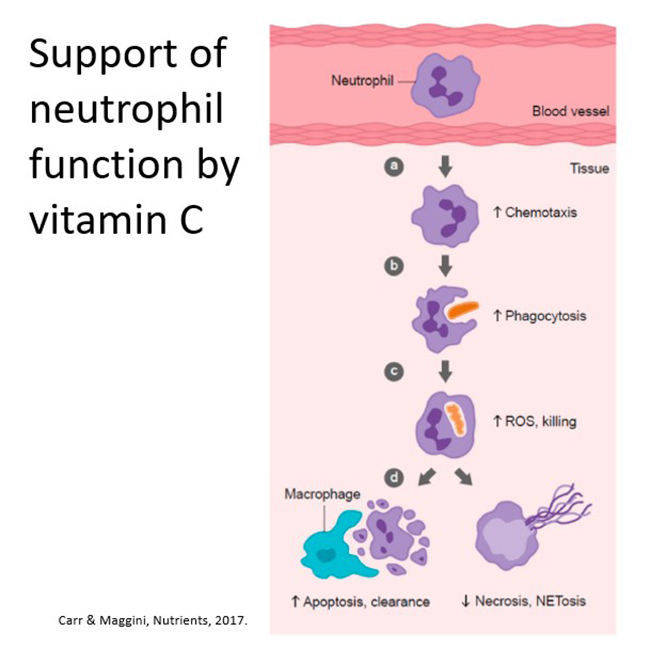
Further reading:
- Vitamin C and immune function. Carr and Maggini. Nutrients, 2017.
This narrative review discusses the various roles that vitamin C plays in the innate and adaptive immune system.
3.7.3.Support of respiratory system
Acute respiratory infections and sepsis can result in the development of acute lung injury which, in its most severe form, is known as acute respiratory distress syndrome (ARDS). In a murine model of sepsis-induced acute lung injury, concurrent administration of 200 mg/kg vitamin C to mice attenuated the resultant lung dysfunction, including decreased lung water and alveolar epithelial permeability and increased alveolar fluid clearance in the animals that received vitamin C. Furthermore, increased expression of iron pumps and channels and increased expression of tight junction and cytoskeletal connector proteins were observed, suggesting enhanced gene transcription in the presence of vitamin C (see figure below).
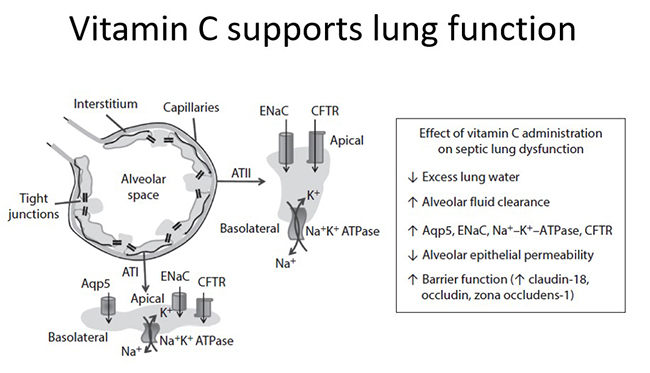
Preclinical research indicated that administration of 200 mg/kg vitamin C to mice attenuated sepsis-induced sequestration of neutrophils, and decreased myeloperoxidase mRNA expression, in the lungs of the treated animals. This was also associated with a lower acute lung injury score and decreased mortality in these animals.
Preclinical research in vitamin C–deficient Gulo knockout mice indicated enhanced NETs in the lungs of septic animals. The levels of these markers were attenuated in vitamin C–sufficient animals or in vitamin C–deficient animals that were administered vitamin C following induction of sepsis.
Case reports have been published that demonstrate dramatic lung clearance in septic patients with acute respiratory distress syndrome of bacterial and viral origins following treatment with intravenous vitamin C. Vitamin C was administered to the patients at a dose of 200 mg/kg/day, and within 1–2 days there was observable clearance of the lung infiltrate upon chest x-ray (see figure below).
Further reading:
- Vitamin C in pneumonia and sepsis. Carr A. Chpt 7 in Vitamin C: new biochemical and functional insights.
This book chapter details the mechanisms of action of vitamin C in sepsis.
3.7.4.Support of cardiovascular system
Vitamin C is well known to facilitate the synthesis of the catecholamines dopamine, norepinephrine, and epinephrine within the sympathetic nervous system and adrenal medulla. These catecholamines are central to the cardiovascular response to severe infection; they increase arterial pressure through binding to α-adrenergic receptors on the smooth muscle cells of the vasculature and can promote increased cardiac contractility and heart rate through binding to β-adrenergic receptors on cardiac muscle.
Vitamin C is a cofactor for the copper-containing monooxygenase dopamine β-hydroxylase that introduces a hydroxyl group onto dopamine to form norepinephrine (see figure below). Epinephrine is subsequently synthesised in the adrenal glands via methylation of the amine group of norepinephrine. Recent research indicates that vitamin C may also stimulate the rate-limiting enzyme tyrosine hydroxylase via recycling the enzyme's cofactor, tetrahydrobiopterin, thus facilitating hydroxylation of L-tyrosine to form the dopamine precursor L-dopa. Some evidence also suggests that vitamin C may enhance the synthesis of tyrosine hydroxylase; this is possibly through its gene-regulatory effects.
Of note, norepinephrine levels are decreased in vitamin C–deficient animal models, particularly in the adrenal glands. Research has also shown that vitamin C is secreted from the adrenal glands as part of the stress response, which could conceivably result in adrenal vitamin C depletion under conditions of sustained stress.
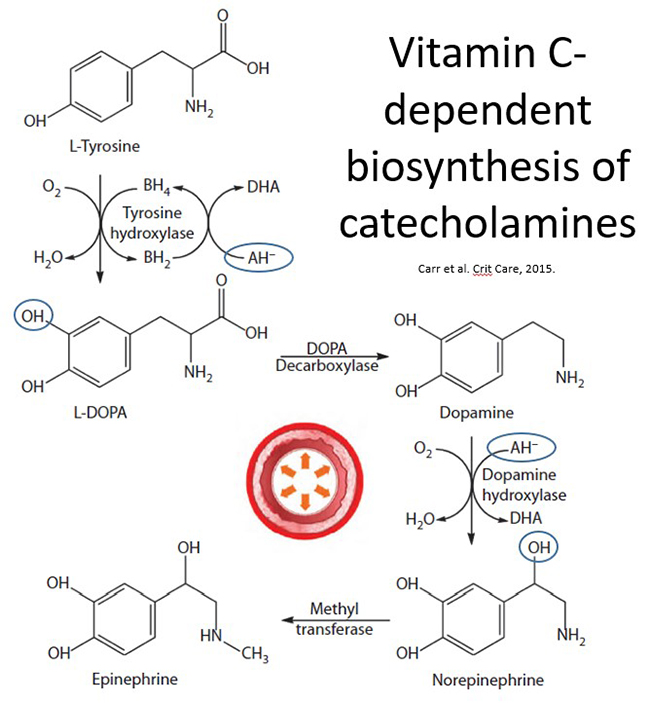
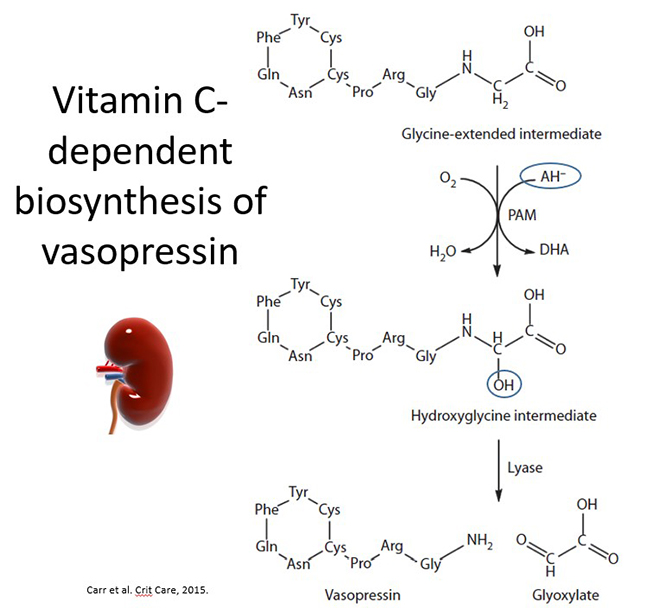
Vitamin C is also a cofactor for the copper-containing enzyme peptidylglycine α-amidating monooxygenase (PAM) that is required for the synthesis of amidated neuropeptide hormones. The carboxy-terminal amine group of amidated peptides is essential for their biological activities. One of these amidated peptide hormones is vasopressin, also known as arginine vasopressin (AVP) or antidiuretic hormone (ADH), which is synthesized in the hypothalamus, post-translationally modified by PAM, and then stored in the posterior pituitary. Vasopressin is secreted in response to decreased blood volume or arterial pressure or increased plasma osmolality. It interacts with specific receptors expressed by vascular smooth muscle cells and kidney collecting ducts to cause vasoconstriction and water retention, respectively.
Vasopressin is synthesized as a pre-prohormone that undergoes sequential cleavage steps to produce pro-vasopressin and finally a glycine-extended precursor. The carboxy-terminal glycine residue of the vasopressin precursor subsequently undergoes posttranslational modification by the vitamin C–dependent enzyme PAM to generate the active carboxy-amidated hormone (see figure below). Support for a connection between vitamin C and vasopressin biosynthesis comes from an animal study, whereby centrally administered vitamin C enhanced circulating levels of vasopressin and induced antidiuresis.
It is also noteworthy that the tissues where catecholamines and vasopressin are synthesized (i.e., the brain and pituitary and adrenal glands) contain the highest levels of vitamin C in the body, indicating that the vitamin plays a vital role in these organs.
Septic shock is normally managed through the administration of catecholamine vasopressors, primarily norepinephrine, to elevate mean arterial pressure to ≥65 mm Hg. Vasopressin administration is also recommended to raise mean arterial pressure to target or to decrease the norepinephrine dose. Based on the evidence presented above, we hypothesised that administration of vitamin C to patients with septic shock may decrease the requirement for exogenously administered vasopressors, through acting as a cofactor for in vivo enzyme-dependent synthesis of norepinephrine and vasopressin (see further reading below).
This hypothesis has been supported by a handful of small trials carried out in low-middle income countries that showed deceased vasopressor and norepinephrine requirements (both dose and duration) following administration of intravenous vitamin C to patients with severe sepsis and septic shock. We were not able to confirm this finding in a small study (n=40) in NZ, but this could be partly due to the difference in vitamin C status of the different populations prior to sepsis, with people from low-middle income countries often exhibiting chronic vitamin C insufficiency prior to the acute deficiency induced by sepsis.
Further reading:
- Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Carr et al. Crit Care, 2015.
This review presents the hypothesis that one of the mechanisms by which vitamin C could be acting in septic patients is through supporting the endogenous biosynthesis of vasopressors.
3.8. Summary of Module 2
- Severe respiratory infections such as pneumonia are one of the major comorbidities and causes of mortality for people with the vitamin C deficiency disease scurvy. Epidemiological evidence has indicated that people with higher vitamin C status had lower risk and mortality from pneumonia.
- Severe infections can significantly deplete vitamin C levels due to enhanced utilization of the vitamin during the infectious process. The extent of depletion depends on the severity of the condition, with critically ill septic patients having the lowest vitamin C levels.
- Prophylactic vitamin C (doses ≥200 mg/day) does not decrease the risk of upper respiratory tract infections, except in adults under enhanced physical stress (>50% decreased risk). However, both the duration and severity of the common cold are decreased with prophylactic and therapeutic (dose-dependent) vitamin C administration.
- Prophylactic vitamin C (doses ≥200 mg/day) can decrease the risk of pneumonia, including in people with influenza. Therapeutic administration to patients with pneumonia can decrease the severity and hospital length of stay (dose-dependent), with a trend towards decreased mortality.
- Up to 40% of patients with pneumonia and sepsis can have deficient levels of vitamin C (≤11 µmol/L) and up to 90% of critically ill septic patients can have hypovitaminosis C (≤23 µmol/L). This is despite the patients receiving recommended enteral/parenteral intakes of the vitamin.
- Critically ill and septic patients have enhanced requirements for vitamin C. Recommended intakes that would normally saturate the plasma of healthy people (i.e. 200-300 mg/day) are insufficient. Instead these patients require parenteral administration of 10 times this amount (i.e. 2-3 g/day) to restore saturating plasma vitamin C levels.
- In trials of critically ill septic patients, IV vitamin C is usually administered in 2-4 doses over the day (total dose of 100 – 200 mg/kg body weight/day for 4-7 days). Hypovitaminosis C can occur in some patients following early cessation of the intervention, suggesting sustained therapy may be required to prevent this from occurring.
- The CITRIS-ALI trial (n=167) showed decreased mortality and increased ICU and hospital-free days. Vitamin C intervention decreased mortality by 81% during the 4-day intervention period. In contrast, the LOVIT trial (n=872) did not show improved mortality/organ dysfunction, although trending in the subgroup of patients who had SARS-CoV2 infection.
- Patients with COVID-19 have comparably low vitamin C levels to those with pneumonia and sepsis. Administration of 24 g/day IV vitamin C to ICU patients with COVID-19 (in Wuhan, China) showed decrease in ICU and hospital mortality in those with SOFA score ≥3. Administration of 8 g/day oral vitamin C to patients with SARS-CoV2 infection (in the USA) decreased duration of symptoms by 1.2 days and increased rate of recovery by 71%.
- Vitamin C may be working via anti-inflammatory mechanisms (decreasing CRP, PCT, IL-6). Vitamin C supports various cell functions in both the innate and adaptive immune systems. The vitamin also supports lung function, likely via its gene regulatory cofactor functions, and supports the cardiovascular system via its biosynthetic cofactor functions (e.g. synthesis of noradrenaline and vasopressin).
3.9. Activity: Self-reflection and feedback
Once you have completed all the Modules of interest to you, please complete the self-reflection and feedback form online or email to anitra.carr@otago.ac.nz. For members of the RNZCGP, please include your MCNZ number and the modules completed so that your CPD credits can be uploaded to the RNZCGP website. A certificate of completion can be emailed to you if required.
© Anitra Carr, 2022. You may not copy, modify, distribute, display, transmit, perform, publish or sell any of the copyrightable material on this website. You may hyperlink to this website but must include the following statement: "This link leads to the website 'Oral and intravenous vitamin C use in health care' provided by the Nutrition in Medicine Research Group at University of Otago, Christchurch."
