This online resource is an up-to-date repository of published and ongoing vitamin C and COVID-19 research studies and related resources.
If you wish to provide relevant resources or receive further information, contact:
Research Professor Anitra Carr
Email anitra.carr@otago.ac.nz
In March 2020, the World Health Organization (WHO) published a 'Coordinated Global Research Roadmap for the 2019 Novel Coronavirus'. In this document vitamin C was highlighted as an adjunctive intervention with biological plausibility to improve the outcome of COVID-19 infected patients (pages 36-7).
Vitamin C and COVID-19 observational studies
Low vitamin C levels can predispose people to viral infections. In addition, viral infections can further decrease vitamin C levels due to enhanced requirements for the vitamin during infections. Below is a summary of COVID-19 and vitamin C observational studies which indicate that patients with COVID-19 have depleted vitamin C status. Definitions: vitamin C deficiency ≤11 µmol/L (<0.2 mg/dL); hypovitaminosis C ≤23 µmol/L (<0.4 mg/dL).
Observational studies
| Study 1 | Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome |
|---|---|
| Date online | 26 August 2020 |
| Location | Barcelona, Spain |
| Cohort | 18 patients with SARS-CoV-2-associated acute respiratory distress syndrome (ARDS) |
| Findings | 17 patients had <1.5 mg/L (<8.5 µmol/L) vitamin C 1 patient had 2.4 mg/L (14 µmol/L) vitamin C |
| Reference | Chiscano-Camón et al. Crit Care. 2020 24(1):522. doi:10.1186/s13054-020-03249-y |
| Study 2 | Serum levels of vitamin C and vitamin D in a cohort of critically Ill COVID-19 patients of a North American community hospital intensive care unit in May 2020: A pilot study |
|---|---|
| Date online | 18 September 2020 |
| Location | Thornton, Colorado, USA |
| Cohort | 21 critically ill COVID-19 patients |
| Findings |
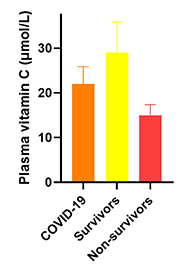 Total cohort (n = 21) mean vitamin C status 22 ± 4 µmol/L (45% deficient, 70% hypovitC) Total cohort (n = 21) mean vitamin C status 22 ± 4 µmol/L (45% deficient, 70% hypovitC)Survivors (n = 11) mean vitamin C status 29 ± 7 µmol/L (40% deficient, 50% hypovitC) Non-survivors (n = 10) mean vitamin C status 15 ± 2 µmol/L (50% deficient, 90% hypovitC) |
| Reference | Arvinte et al. Med Drug Discov. 2020 8:100064. doi:10.1016/j.medidd.2020.100064 |
| Study 3 | Vitamin C supplementation is necessary for patients with coronavirus disease: An ultra-high-performance liquid chromatography-tandemmass spectrometry finding |
|---|---|
| Date online | 27 January 2021 |
| Location | Shanghai, China |
| Cohort | 31 COVID-19 patients (+/- IV vitamin C) and 51 healthy controls |
| Findings |
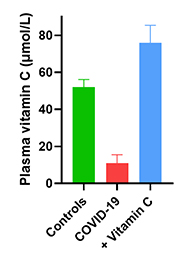 6 COVID-19 patients (no IV vitamin C): mean plasma vitamin C level 2 mg/dl (11 µmol/L) 6 COVID-19 patients (no IV vitamin C): mean plasma vitamin C level 2 mg/dl (11 µmol/L)25 COVID-19 patients given 100 mg/kg/d IV vitamin C: mean plasma vitamin C level 13.5 mg/L (76 µmol/L) 51 healthy controls: mean plasma vitamin C level 9.2 mg/L (52 µmol/L) |
| Reference | Xing et al. J Pharm Biomed Anal. 2021, 196:113927. doi: 10.1016/j.jpba.2021.113927 |
| Study 4 | Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria |
|---|---|
| Date online | 1 February 2021 |
| Location | Jigawa, Northwestern Nigeria |
| Cohort | 50 COVID-19 symptomatic patients 21 healthy controls |
| Findings | Patients had 0.33 mg/dL (19 µmol/L) vitamin C Controls had 0.44 mg/dL (25 µmol/L) vitamin C (p <0.001) |
| Notes | Oxidative stress markers were elevated in the patients relative to controls and correlated with vitamin C in the patients (r = -0.605, p = 0.004) |
| Reference | Muhammad et al. SAGE Open Medicine. 2021 9:1-8. doi/10.1177/2050312121991246 |
| Study 5 | Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study |
|---|---|
| Date online | 7 February 2021 |
| Location | Liège, Belgium |
| Cohort | 9 ICU patients with severe COVID-19 pneumonia |
| Findings | Patients had 3.9 (3.1–6.1) µg/mL or 22 (18-35) µmol/L vitamin C Reference rage 6.2–15.2 µg/mL or 35-86 µmol/L (p = 0.004) |
| Notes | These low concentrations were despite the patients receiving 124 (95-172) mg/day of vitamin C in their liquid nutrition |
| Reference | Pincemail et al. Antioxidants. 2021 10(2):257. doi: 10.3390/antiox10020257 |
| Study 6 | Evaluation of nutritional status in pediatric patients diagnosed with Covid-19 infection |
|---|---|
| Date online | 11 May 2021 |
| Location | Ankara, Turkey |
| Cohort | 49 paediatric patients (aged 1 month to 18 years), diagnosed with COVID-19 then hospitalized |
| Findings | 17% of the patients had vitamin C deficiency |
| Reference | Molla et al. Clin Nutr ESPEN. 2021. doi: 10.1016/j.clnesp.2021.04.022. |
| Study 7 | COVID‑19: Up to 82% critically ill patients had low Vitamin C values |
|---|---|
| Date online | 9 July 2021 |
| Location | Barcelona, Spain |
| Cohort | 67 critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS) |
| Findings | Mean vitamin C concentration was 8 ± 3 µmol/L (0.14 ± 0.05 mg/dL); range of <6 – 61 µmol/L (<0.10 – 1.08 mg/dL) 55 patients (82%) had values <23 µmol/L (<0.40 mg/dL) 12 patients (18%) had values <6 µmol/L (<0.10 mg/dL) |
| Reference | Tomasa‑Irriguible and Bielsa‑Berrocal. Nutr Res. 2021 20(1):66. doi: 10.1186/s12937-021-00727-z. |
| Study 8 | COVID‑19: Up to 82% critically ill patients had low Vitamin C values |
|---|---|
| Date online | 15 August 2022 |
| Location | St. Gallen, Switzerland |
| Cohort | 74 COVID-19 patients 8 unidentified volunteers |
| Findings | COVID-19 patients had significantly lower plasma ascorbate levels (2.8 [0.5, 15] µmol/L) than the controls (47 [44, 52] µmol/L; p < 0.001) Survival analysis showed that plasma AA < 11.4 µM was associated with a lengthy hospitalization and a high risk of death. |
| Reference | Sinnberg et al. Antioxidants (Basel). 2022;11(8):1580. doi: 10.3390/antiox11081580. |
| Study 9 | Correlation Between Plasma Vitamin C Concentration and COVID-19 Outcomes among Patients Seen at a Major Hospital in the United Arab Emirates |
|---|---|
| Date online | 12 February 2022 |
| Location | Abu Dhabi, United Arab Emirates |
| Cohort | 67 COVID-19 patients |
| Findings | 58% suffered from vitamin C deficiency Low vitamin C concentrations were associated with age, hypertension, diabetes, the presence of pneumonia, and inflammation. |
| Reference | Hafez et al. Int J MCH AIDS. 2022;11(2):e608. doi: 10.21106/ijma.608. |
| Study 10 | Vitamin C as a Potential Interplaying Factor between Obesity and COVID-19 Outcome |
|---|---|
| Date online | 28 December 2022 |
| Location | Cairo, Egypt |
| Cohort | 63 COVID-19 patients |
| Findings |
There was no significant difference in vitamin C levels among patients in different BMI categories (p > 0.05) and vitamin C did not affect the risk of COVID-19 severity or mortality across BMI categories (p > 0.05). Rate of viral clearance was significantly lower in obese patients who also had low vitamin C levels (p < 0.05). Note: the authors did not report vitamin C concentrations. |
| Reference | Hafez et al. Healthcare (Basel). 2022 Dec 28;11(1):93. doi: 10.3390/healthcare11010093. |
| Study 11 | Baseline serum vitamin A and vitamin C levels and their association with disease severity in COVID-19 patients |
|---|---|
| Date online | 13 February 2023 |
| Location | Istanbul, Turkey |
| Cohort | 53 COVID-19 patients 26 healthy volunteers |
| Findings |
Vitamin C levels were significantly lower relative to healthy controls (p=0.007). Inverse correlations between vitamin C levels and length of hospital stay (r=-0.478; p<0.001) and chest CT severity score (r=-0.734: p<0.001). |
| Reference | Yilmaz et al. Acta Biomed. 2023 Feb 13;94(1):e2023007. doi: 10.23750/abm.v94i1.13655. |
Vitamin C and COVID-19 intervention trials
Randomised controlled trials (RCTs) of patients with respiratory infections, pneumonia and sepsis have indicated that vitamin C administration may be able to improve some patient outcomes. Critically ill patients with sepsis require intravenous administration of gram doses of vitamin C to normalise their plasma vitamin C levels. Below is a summary of vitamin C and COVID-19 intervention trials which indicate that some symptoms of COVID-19 patients may be improved with vitamin C administration.
Intravenous vitamin C – randomised controlled trials
| RCT 1 | Pilot trial of high-dose vitamin C in critically ill COVID-19 patients |
|---|---|
| Date online | 9 August 2020 |
| Location | Hubei, China (multicentre) |
| Trial type | Randomised placebo-controlled trial |
| Cohort | 54 critically ill COVID-19 patients (27 and 29 per group) |
| Intervention | 24 g/day IV vitamin C (12 g/12 hours) for 7 days (or placebo) |
| Findings |
|
| Notes | Trial was stopped early due to lack of patients |
| Reference | Zhang et al. Ann Intensive Care, 2021, 11, 5 |
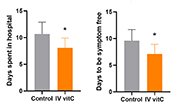
| RCT 2 | The role of vitamin C as adjuvant therapy in COVID-19 |
|---|---|
| Date online | 30 November 2020 |
| Location | Karachi, Pakistan |
| Trial type | Randomised controlled trial (open label) |
| Cohort | 150 severe COVID-19 patients (75 per group) |
| Intervention | 50 mg/kg bodyweight/day IV vitamin C (or standard therapy alone) |
| Findings |
|
| Reference | Kumari et al. Cureus 2020 12(11): e11779. doi:10.7759/cureus.11779 |
| RCT 3 | Safety and effectiveness of high‑dose vitamin C in patients with COVID‑19: a randomized open‑label clinical trial |
|---|---|
| Date online | 11 February 2021 |
| Location | Tehran, Iran |
| Trial type | Randomised controlled trial (open-label) |
| Cohort | 60 patients with COVID-19 (30 per group) |
| Intervention | 6 g/d IV vitamin C (or standard care) for 5 days |
| Findings | ↓ body temperature on 3rd day of hospitalization (p = 0.001) ↑ peripheral capillary oxygen saturations (SpO2) on 3rd day of hospitalization (p = 0.014) Comparable SpO2 levels (oxygen saturation) at discharge ↑ length of hospitalization (8.5 days vs. 6.5 days, p = 0.028). No difference in length of intensive care unit (ICU) stay or mortality No adverse events were reported |
| Reference | JamaliMoghadamSiahkali et al. Eur J Med Res. 2021, 26(1):20. doi: 10.1186/s40001-021-00490-1 |
| RCT 4 | An investigation into the effects of intravenous vitamin C on pulmonary CT findings and clinical outcomes of patients with COVID 19 pneumonia A Randomized Clinical Trial |
|---|---|
| Date online | 8 November 2021 |
| Location | Tehran, Iran |
| Trial type | Randomised controlled trial (open-label) |
| Cohort | 50 patients with moderate to severe COVID-19 |
| Intervention | IV vitamin C at 2 g every 6 hours (8 g/day) for 5 days (n = 18) or standard care (n = 26) |
| Findings | ↑ oxygen saturation (p = 0.02) |
| Reference | Tehrani et al. Urol J. 2021 doi: 10.22037/uj.v18i.6863 |
| RCT 5 | Therapies to prevent progression of COVID-19, including hydroxychloroquine, azithromycin, zinc, and vitamin D3 with or without intravenous vitamin C: An international, multicenter, randomized trial |
|---|---|
| Date online | 25 November 2021 |
| Location | Turkey (7 hospitals) |
| Trial type | Multicenter, randomised, open-label study |
| Cohort | 237 hospitalised patients with COVID-19 |
| Intervention | IV vitamin C (50 mg/kg every six hours on day 1, followed by 100 mg/kg every six hours (average: 28 g/day) for seven days + hydroxychloroquine, azithromycin, zinc, and vitamin D3 (n = 162) vs hydroxychloroquine, azithromycin, zinc, and vitamin D3 (n = 75) |
| Findings | IVC therapy contributed to a quicker recovery |
| Reference | Ried et al. Cureus. 2021. doi: 10.7759/cureus.19902 |
| RCT 6 | Pharmacologic Ascorbic Acid as Early Therapy for Hospitalized Patients with COVID-19: A Randomized Clinical Trial |
|---|---|
| Date online | 19 March 2022 |
| Location | Philadelphia, USA |
| Trial type | Randomised controlled trial |
| Cohort | 66 patients with COVID-19 requiring supplemental oxygen |
| Intervention | Escalating doses of IV vitamin C plus standard of care (n = 44) vs standard of care alone (n = 22) |
| Findings | Overall clinical improvement at 72 h was not achieved, however, point estimates for the composite outcome and its individual components of decreased use of supplemental oxygen, decreased use of bronchodilators, and the time to discharge were all favourable for the treatment arm, particularly at earlier time points (see figures). |
| Reference | Coppock et al. Life (Basel). 2022;12(3):453. doi: 10.3390/life12030453 |
| RCT 7 | Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit |
|---|---|
| Date online | 15 June 2022 |
| Location | Canada, France, New Zealand |
| Trial type | Randomised placebo-controlled trial |
| Cohort | 63 patients with SARS-CoV-2 infection (within a total cohort of 872 patients with sepsis) |
| Intervention | IV vitamin C (50 mg/kg body weight) every 6 hours for up to 96 hours (n = 37) vs matched placebo (n = 26) |
| Findings | A non-significant decrease in risk of death or persistent organ dysfunction in the vitamin C group (RR 0.81, 95% CI 0.57–1.16). |
| Reference | Lamontagne et al. N Engl J Med. 2022;386(25):2387-2398. doi: 10.1056/NEJMoa2200644. |
| RCT 8 | Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial |
|---|---|
| Date online | 30 August 2022 |
| Location | Patna, Bihar, India |
| Trial type | Double-blind randomized placebo-controlled trial |
| Cohort | 60 patients with moderate to severe COVID-19 |
| Intervention | IV vitamin C - 1 gram 8 hourly (3 g/day) (n = 30) or IV placebo (n = 30) for four days |
| Findings | 10 (33%) died in intervention group vs 13 (43%) in placebo group (P > 0.05) |
| Reference | Kumar et al. J Family Med Prim Care. 2022;11(8):4758-4765. doi: 10.4103/jfmpc.jfmpc_2437_21. |
| RCT 9 |
High-dose Intravenous Vitamin C in Early Stages of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Double-blind, Randomized, Controlled Clinical Trial |
|---|---|
| Date online | 12 December 2022 |
| Location | Tehran, Iran |
| Trial type | Double-blind randomized placebo-controlled trial |
| Cohort | 74 patients with moderate to severe COVID-19 (37 per group) |
| Intervention | 12 g/day IV vitamin C for 4 days (or placebo) |
| Findings | No significant difference in SOFA score or 28-day mortality (p > 0.05) |
| Reference | Labbani-Motlagh et al. J Res Pharm Pract. 2022 Dec 14;11(2):64-72. doi: 10.4103/jrpp.jrpp_30_22. |
Intravenous vitamin C - retrospective cohort studies (11 studies)
| Study 1 | The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study |
|---|---|
| Date online | 26 February 2021 |
| Location | Xi'an, Shaanxi, China |
| Trial type | Retrospective cohort |
| Cohort | 76 patients with COVID-19 |
| Intervention | Loading dose of 6 g intravenous infusion per 12 hr on the first day, and 6 g once for the following 4 days (n=46) or standard therapy group (n=30) |
| Findings |
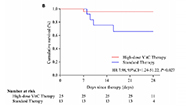
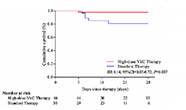 Risk of 28-day mortality was reduced (HR=0.14, 95% CI, 0.03-0.72) Risk of 28-day mortality was reduced (HR=0.14, 95% CI, 0.03-0.72)Oxygen support status was improved compared with standard therapy (64% vs 36%) No adverse safety events were associated with high-dose vitamin C therapy |
| Notes |
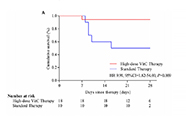 Mortality differences were even more pronounced for (A) those with severe COVID-19 (HR= 9.91, 95% CI, 1.82-54.00) and (B) those aged >60 years (HR=7.98, 95% CI, 1.24-51.22). Mortality differences were even more pronounced for (A) those with severe COVID-19 (HR= 9.91, 95% CI, 1.82-54.00) and (B) those aged >60 years (HR=7.98, 95% CI, 1.24-51.22).
|
| Reference | Gao et al. Aging. 2021:13. doi: 10.18632/aging.202557. |
| Study 2 | High Dose Intravenous Vitamin C for Preventing The Disease Aggravation of Moderate COVID-19 Pneumonia. A Retrospective Propensity Matched Before-After Study |
|---|---|
| Date online | 22 April 2021 |
| Location | Shanghai, China |
| Trial type | Retrospective propensity matched |
| Cohort | 110 patients with moderate COVID-19 pneumonia (55 per group) |
| Intervention | 100 mg/kg/day IV vitamin C for 7 days vs standard care |
| Findings | ↓ number of patients progressing to severe type (4/55 vs 12/55; RR 0.28 [0.08, 0.93], P = 0.03) ↓ duration (P < 0.001) and ↓ incidence (2/21 vs 10/22, P = 0.08) of systemic inflammation (SIRS) ↓ C-reactive protein levels (P = 0.05), ↓ activated partial thromboplastin (blood clotting) time (P = 0.02), ↑ CD4+ (helper) T cells (P = 0.04) No Effect on time to negative nucleic acid load |
| Reference | Zhao et al. Frontiers Pharmacol, 2021, 12:638556. |
| Study 3 | Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia |
|---|---|
| Date online | 11 May 2021 |
| Location | Ankara, Turkey |
| Trial type | Retrospective |
| Cohort | 232 patients with severe acute respiratory syndrome due to COVID-19 infection |
| Intervention | 2 g/day IV vitamin C initiated within a median duration of 3 days after admission (n=153) vs no vitamin C (n=170) |
| Findings | Those in the VC group were not significantly different in terms of the length of hospital stay (p=0.05), re-admission rate (p=0.943), admission to intensive care, need for advanced oxygen support (p=0.488), need for advanced medical treatment (p<0.001), and mortality (p=0.52) No adverse effects associated with the use of IV vitamin C treatment were recorded. |
| Reference | Suna et al. Med Clin (Barc). 2021;S0025-7753(21)00252-9. doi: 10.1016/j.medcli.2021.04.010. |
| Study 4 | Use of Intravenous Vitamin C in Critically Ill Patients With COVID-19 Infection |
|---|---|
| Date online | 8 June 2021 |
| Location | New York, United States of America |
| Trial type | Retrospective observational cohort study with propensity score matching |
| Cohort | ICU patients with COVID-19 infection |
| Intervention | 1.5 grams IV vitamin C every 6 hours for up to 4 days (n = 8) vs matched patients (n = 24) |
| Findings | Patients in the IV vitamin C group had higher rates of hospital mortality [7 (88%) vs. 19 (79%), P = 0.049] and mean SOFA scores post-treatment (12.4 ± 2.8 vs. 8.1 ± 3.5, P < 0.005). There was no difference in the daily vasopressor requirement or in ICU length of stay between the treatment and control groups. |
| Reference | Li et al. J Pharm Pract. 2021;8971900211015052. doi: 10.1177/08971900211015052. |
| Study 5 | High-dose intravenous vitamin C attenuates hyperinflammation in severe coronavirus disease 2019 |
|---|---|
| Date online | 26 June 2021 |
| Location | Wuhan, China |
| Trial type | Retrospective cohort |
| Cohort | 236 patients with severe COVID-19 |
| Intervention | Intravenous vitamin C (IVC) at 100 mg/kg body weight every 6 hours on the first day, then 100 mg/kg body weight every 12 h for the next 5 days (n=85) or standard therapy group (n=151) |
| Findings | IVC intervention was associated with reduced levels of inflammatory markers (C-reactive protein, interleukin-6, tumor necrosis factor-α) |
| Reference | Xia et al. Nutrition 2021, 91-92:111405. doi: 10.1016/j.nut.2021.111405.g. |
| Study 6 | The use of vitamin C in the intensive care unit during the COVID-19 pandemic |
|---|---|
| Date online | July 2021 |
| Location | Bursa, Turkey |
| Trial type | Retrospective cohort |
| Cohort | 160 ICU patients with COVID-19 |
| Intervention | IV vitamin C at 3x 2g (n=32) or no vitamin C (n=128) |
| Findings | No differences observed between groups for inflammatory parameters (e.g. C-reactive protein, procalcitonin, D dimer), length of stay or mortality. |
| Reference | Özgünay et al. Eur Res J 2021. doi:10.18621/eurj.938778 |
| Study 7 | High-dose vitamin C ameliorates cardiac injury in COVID-19 pandemic: a retrospective cohort study |
|---|---|
| Date online | 9 September 2021 |
| Location | Wuhan, China |
| Trial type | Retrospective cohort |
| Cohort | 113 severe and critical COVID-19 patients with cardiac injury |
| Intervention | Intravenous vitamin C (IVC) at 100 mg/kg body weight every 6 hours on the first day, then 100 mg/kg body weight every 12 h for the next 5 days (n = 51) or standard therapy group (n = 62) |
| Findings | More patients in ameliorated cardiac injury group received IVC (53 vs 33%, p = 0.035) IVC was associated with ameliorated cardiac injury independent of other medications (OR 2.42 [1.02, 5.73], p = 0.04) IVC was associated with reduced levels of inflammatory markers (C-reactive protein, interleukin-6, interleukin-8, tumor necrosis factor-α) at day 21 of hospitalisation (p <0.05) |
| Reference | Xia et al. Aging 2021, 13. doi: 10.18632/aging.203503. |
| Study 8 | Effect of Vitamin C on Clinical Outcomes of Critically Ill Patients With COVID-19: An Observational Study and Subsequent Meta-Analysis |
|---|---|
| Date online | 11 February 2022 |
| Location | Athens, Greece |
| Trial type | Retrospective cohort |
| Cohort | 113 adult COVID-19 patients with critical COVID-19 |
| Intervention | Intravenous vitamin C (1-3 g/day; n = 10) No vitamin C (n = 103) |
| Findings | ICU mortality 20% (2/10) in vitamin C group vs 48% (49/103) in control group (P = 0.1) Vasopressor free days 9 vs 0 for vitamin C group vs control group (P = 0.3) Continuous renal replacement therapy free days 26 vs 19 for vitamin C group vs control group (P = 0.6) |
| Reference | Gavrielatou et al. Front Med (Lausanne). 2022;9:814587. doi: 10.3389/fmed.2022.814587. |
| Study 9 | No significant benefit of moderate-dose vitamin C on severe COVID-19 cases |
|---|---|
| Date online | 22 September 2021 |
| Location | Wuhan, China |
| Trial type | Retrospective cohort |
| Cohort | 397 adult patients with severe COVID-19 |
| Intervention | Intravenous vitamin C (2-4 g/day; n = 70) No vitamin C (n = 327) |
| Findings | No difference in mortality between groups No difference in clinical improvement between groups |
| Reference | Zheng et al. Open Med (Wars) 2021, 16(1):1403-1414. doi: 10.1515/med-2021-0361 |
| Study 10 | High-dose intravenous vitamin C decreases rates of mechanical ventilation and cardiac arrest in severe COVID-19 |
|---|---|
| Date online | 29 March 2022 |
| Location | Missouri, USA |
| Trial type | Retrospective cohort |
| Cohort | 100 patients with severe COVID-19 |
| Intervention | Intravenous vitamin C (3 g every 6 h for 7 days; n = 25) Control - no vitamin C (n = 75) |
| Findings | Average time to death was significantly longer for HDIVC patients (22.9 days versus 13.7 days for control patients; P = 0.0139). |
| Reference | Hess et al. Intern Emerg Med. 2022;1-10. doi: 10.1007/s11739-022-02954-6. |
| Study 11 | Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia |
|---|---|
| Date online | 22 April 2022 |
| Location | Ankara, Turkey |
| Trial type | Retrospective cohort |
| Cohort | 323 patients with severe COVID-19 |
| Intervention | Intravenous vitamin C (2 g/day; n = 153) Control - no vitamin C (n = 170) |
| Findings | Vitamin C group relative to control group: length of hospital stay (p = 0.05), re-admission rate (p = 0.9), admission to intensive care (ns), need for advanced oxygen support (p = 0.5), need for advanced medical treatment (p < 0.001), and mortality (p = 0.5) |
| Notes | V vitamin C was relatively low dose (2 g/day) and was administered a median of 3 days after admission. Duration of vitamin C administration was not stated. |
| Reference | Suna et al. Med Clin. 2022;158(8):356-360. doi: 10.1016/j.medcle.2021.04.027 2022;1-10. |
Oral vitamin C - randomised controlled trials (3 RCTs)
| RCT 1 | Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial |
|---|---|
| Date online | 12 February 2021 |
| Location | Ohio and Florida, USA |
| Trial type | Randomised controlled trial (open-label) |
| Cohort | 214 patients with SARS-CoV-2 infection (48 - 58 per group) |
| Intervention | 8 g/d oral vitamin C or 50 mg/d zinc gluconate or both vitamin C and zinc gluconate or standard care for 10 days |
| Findings | Non-significant decrease in number of days to reach 50% reduction in symptoms of approx. one day for treatment groups compared with standard care: Standard care group 6.7 (± 4.4) days Vitamin C group 5.5 (± 3.7) days Zinc gluconate group 5.9 (± 4.9) days Both vitamin C and zinc gluconate group 5.5 (± 3.4) days |
| Notes |
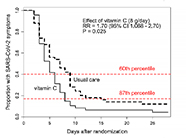 Trial was stopped early as interventions assumed to be ineffective (although the difference between the vitamin C and standard care groups was greater than originally anticipated by the authors). Trial was stopped early as interventions assumed to be ineffective (although the difference between the vitamin C and standard care groups was greater than originally anticipated by the authors).Independent statistical analysis of the data has indicated that vitamin C increased the rate of recovery by 71% (P = 0.036) |
| Reference | Thomas et al. JAMA Network Open 2021 4(2):e210369. doi:10.1001/jamanetworkopen.2021.0369 |
| RCT 2 | The Effect of Vitamin C on Pathological Parameters and Survival Duration of Critically Ill Coronavirus Disease 2019 Patients: A Randomized Clinical Trial |
|---|---|
| Date online | 15 December 2021 |
| Location | Rasht, Iran |
| Trial type | Double blind randomised controlled trial |
| Cohort | 120 critically ill patients infected with COVID-19 (31 intervention group, 69 control group) |
| Intervention | 500 mg/day oral vitamin C for 14 days |
| Findings | Higher mean survival duration compared with control group (8 vs. 4 days, p < 0.01) Linear association between the number of days of vitamin C intake and survival duration Lower serum potassium levels, but no difference in other blood parameters |
| Reference | Majidi et al. Front Immunol. 2021;12:717816. doi: 10.3389/fimmu.2021.717816. |
| RCT 3 | A Pilot of a Randomized Control Trial of Melatonin and Vitamin C for Mild-to-Moderate COVID-19 |
|---|---|
| Date online | July-August 2022 |
| Location | Lancaster County, Pennsylvania |
| Trial type | Randomized, double-blind, placebo-controlled trial |
| Cohort | 98 patients with mild-to-moderate symptoms of COVID-19 infection |
| Intervention | vitamin C 1000 mg/d orally (n = 32) or melatonin 10 mg/d orally (n = 32) or placebo (n = 34), orally for 14 days |
| Findings | Vitamin C 1000 mg once daily had no effect on symptom progression or quality of life impact. |
| Reference | Fogleman et al. J Am Board Fam Med. 2022;35(4):695-707. doi: 10.3122/jabfm.2022.04.210529. |
Oral vitamin C - retrospective cohort studies (1 study)
| Study 1 | Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: a propensity score matched study |
|---|---|
| Date online | 3 September 2021 |
| Location | Riyadh, Saudi Arabia |
| Study type | Retrospective propensity score matched |
| Cohort | 296 critically ill patients with COVID-19 |
| Intervention | 1000 mg/d oral vitamin C for 11 (7-18) days (n = 148 patients) |
| Findings |
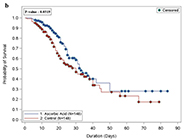 No association of low dose oral vitamin C with in hospital or 30-day mortality, or acute kidney injury, liver injury, respiratory failure/mechanical ventilation No association of low dose oral vitamin C with in hospital or 30-day mortality, or acute kidney injury, liver injury, respiratory failure/mechanical ventilationLonger ICU and hospital length of stay observed for vitamin C group Decreased incidence of thrombosis in vitamin C group (6 vs 13%; OR 0.42 [0.18 – 0.94) p = 0.03) |
| Reference | Sulaiman et al. Sci Rep. 2021; 11(1):17648. doi: 10.1038/s41598-021-96703-y. |
Vitamin C intake or status and COVID-19 risk
| Study 1 | Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study |
|---|---|
| Date online | 16 February 2021 |
| Location | Bhubaneswar, India |
| Study type | Case control |
| Cohort | 372 health-care workers who tested positive (cases) or negative (controls) |
| Intervention | 67 took prophylactic vitamin C at 500 mg/day (or twice daily) vs 305 who did not |
| Findings | Vitamin C prophylaxis was not associated with SARS-CoV-2 infection (OR 0.71, 95% CI, 0.40–1.26) |
| Reference | Behera et al. PLoS ONE 16(2): e0247163. |
| Study 2 | The role of vitamin C in pneumonia and COVID-19 infection in adults with European ancestry: a Mendelian randomisation study |
|---|---|
| Date online | 30 August 2021 |
| Location | Chinese study |
| Study type | Genome-wide association study (GWAS) |
| Cohort | 52,018 people of European ancestry |
| Methodology | Used single nucleotide polymorphisms (SNPs) that were associated with circulating levels of vitamin C (note: they did not use measured vitamin C levels) |
| Findings | Genetically predicted circulating levels of vitamin C was not associated with susceptibility to severe COVID-19, COVID-19 hospitalisation, any COVID-19 infection nor pneumonia. |
| Comments | There are significant issues with using genetically predicted vitamin C concentrations – see commentary by Hemilä and Chalker, Eur J Clin Nutr. 2022. 1-2. doi: 10.1038/s41430-022-01091-9 |
| Reference | Hui et al. Eur J Clin Nutr 2021 1-4. doi: 10.1038/s41430-021-00993-4 |
| Study 3 |
Relationship Between Plasma Vitamin C and COVID-19 Susceptibility and Severity: A Two-Sample Mendelian Randomization Study |
|---|---|
| Date online | 9 March 2022 |
| Location | Chinese study |
| Study type | Genome-wide association study (GWAS) |
| Cohort | 52,018 people of European ancestry |
| Methodology | Mendelian randomization analysis was conducted to examine the effect of selected single nucleotide polymorphisms and COVID-19 susceptibility, hospitalization, disease severity (note: they did not use measured vitamin C levels) |
| Findings | Genetic predisposition to the levels of plasma Vitamin C was not associated with COVID-19 susceptibility, hospitalization and severity. |
| Comments | There are significant issues with using genetically predicted vitamin C concentrations – see commentary by Hemilä and Chalker, Eur J Clin Nutr. 2022. 1-2. doi: 10.1038/s41430-022-01091-9 |
| Reference | Chen et al. Front Med (Lausanne). 2022;9:844228. doi: 10.3389/fmed.2022.844228 |
| Study 4 | Impact of Zinc, Vitamins C and D on Disease Prognosis among Patients with COVID-19 in Bangladesh: A Cross-Sectional Study |
|---|---|
| Date online | 26 November 2022 |
| Location | Bhubaneswar, India |
| Study type | Case control |
| Cohort | 962 participants |
| Methodology | Taking vitamin and/or mineral supplements |
| Findings | Taking vitamin C only decreased infection rate 0.34 (0.042–0.57) p = 0.003 and infection severity 0.54 (0.01–0.92) p = 0.001. |
| Reference | Sharif et al. Nutrients. 2022;14(23):5029. doi: 10.3390/nu14235029. |
Registered vitamin C and COVID-19 clinical trials
Registered vitamin C and COVID-19 clinical trials can be found at clinicaltrials.gov and the International Clinical Trials Registry Platform. Some of these trials are testing vitamin C as a prophylactic (preventative), while others are testing it as an adjunctive therapy/treatment (i.e. in addition to standard care). Some of these trials are using vitamin C alone (monotherapy), while others are using combination therapies. Some are using oral vitamin C and others intravenous vitamin C administration.
Published study protocols
- Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial.
- Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial
- High-dose vitamin C intravenous infusion in the treatment of patients with COVID-19: A protocol for systematic review and meta-analysis
Vitamin C and COVID-19 in clinical practice
Many clinicians and clinical teams worldwide are administering vitamin C to their COVID-19 patients. Some protocols use vitamin C monotherapy in addition to standard care, others as part of combination therapies, for both prophylaxis and treatment.
Clinical protocols
- MATH+ Hospital Treatment Protocol for Covid-19. See also review by Marik, Expert Rev Anti Infect Ther, 2020.(doi: 10.1080/14787210.2020.1808462)
- I-MASK+ Prophylaxis & Early Outpatient Treatment Protocol for COVID-19
- Development and implementation of a COVID-19 near real-time traffic light system in an acute hospital setting (page 4)
- Expert consensus on comprehensive treatment of COVID-19 in Shanghai
Vitamin C and COVID-19 in case reports
Case reports do not have untreated patients as comparators so cannot provide definitive evidence that vitamin C is exerting any additional benefit over standard therapy alone.
Case reports with positive outcomes
- Vitamin C and COVID-19: An Orthomolecular Perspective on Physiological Mechanisms
- Intravenous Vitamin C and an Orthomolecular Protocol as Therapy for COVID19: A Case Report
- High Dose Intravenous Vitamin C as Adjunctive Therapy for COVID-19 Patients with Cancer: Two Cases
- Unusual early recovery of a critical COVID-19 patient after administration of intravenous vitamin C
- The use of IV vitamin C for patients with COVID-19: a case seriesdoi: 10.1080/14787210.2020.1794819
- Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study
- Reversal of the pathophysiological responses to gram-negative sepsis by megadose vitamin C
Case reports with negative outcomes
- Novel coronavirus 2019 (COVID-19): A case report and review of treatments
- Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19
- Kidney transplant dysfunction in a patient with COVID - 19 infection: role of concurrent Sars-Cov 2 nephropathy, chronic rejection and vitamin C-mediated hyperoxalosis: case report
Evidence for mechanisms of action
Many mechanisms of action have been proposed for vitamin C against SARS-CoV-2 and COVID-19, based on previous research with similar viruses and respiratory infections, e.g. anti-viral, anti-inflammatory, anti-oxidant, and immunomodulatory. Below are in vitro (laboratory-based) and in vivo (clinical) studies that have been carried out with SARS-CoV-2 and COVID-19 patients.
In vitro (laboratory-based) studies
- Vitamin C (in the form of magnesium ascorbate) binds to a key protease in the virus, Mpro (Kumar et al. ResearchSquare preprint, 2020)
In vivo (clinical) studies
Intravenous vitamin C administration to COVID-patients:
- Decreased interleukin-6 (IL-6), an inflammatory biomarker (Zhang et al. Ann Intensive Care, 2021)
- Associated with decreased C-reactive protein (an inflammatory marker), decreased activated partial thromboplastin (blood clotting) time, and increases CD4+ (helper) T cells (Zhao et al. Frontiers Pharmacol, 2021)
- Associated with decreased inflammatory markers C-reactive protein (CRP), interleukin-6 (IL-6), and tumour necrosis factor-α (TNFα) (Xia et al. Nutrition, 2021 and Xia et al. Aging, 2021)
Meta-analyses and review articles
Many review articles and commentaries have been written about vitamin C and COVID-19. Published articles that have specifically focused on vitamin C are shown below. Meta-analyses, which combine the outcomes from multiple studies, are now also becoming available.
Published meta-analyses
- Association of Vitamin C Treatment with Clinical Outcomes for COVID-19 Patients: A Systematic Review and Meta-Analysis
- Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis
- Impact of high-dose vitamin C on the mortality, severity, and duration of hospital stay in COVID-19 patients: A meta-analysis
- Effect of Vitamin C on Clinical Outcomes of Critically Ill Patients With COVID-19: An Observational Study and Subsequent Meta-Analysis
- The effectiveness of high-dose intravenous vitamin C for patients with coronavirus disease 2019: A systematic review and meta-analysis
- Vitamin C and COVID-19 treatment: A systematic review and meta-analysis of randomized controlled trials
- Outcomes in vitamin C studies (online meta-analysis)
- Intravenous vitamin C use and risk of severity and mortality in COVID-19: A systematic review and meta-analysis
Published review articles
- The Role of Vitamin C in Human Immunity and Its Treatment Potential Against COVID-19: A Review Article
- Unwinding the potentials of vitamin C in COVID-19 and other diseases: An updated review
- The Role of Vitamin C: From Prevention of Pneumonia to Treatment of Covid-19 (Note: this review is not very well written)
- Role of high dose vitamin C in management of hospitalised COVID-19 patients: A minireview
- Intravenous Ascorbic Acid and Lung Function in Severely Ill COVID-19 Patients
- The Variable Nature of Vitamin C-Does It Help When Dealing with Coronavirus?
- Vitamin C and its therapeutic potential in the management of COVID19
- A possible role for ascorbic acid in COVID-19
- Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C
- Role of vitamin C in preventing of COVID-19 infection, progression and severity
- High-Dose Vitamin C Supplementation as a Legitimate Anti-SARS-CoV-2 Prophylaxis in Healthy Subjects-Yes or No?
- COVID-19, oxidative stress, and male reproductive dysfunctions: Vitamin C as a potential remedy?
- The effects of vitamin C on the multiple pathophysiological stages of COVID-19
- The protective role of vitamin C in the management of COVID
- Vitamin C intervention for critical COVID-19: A pragmatic review of the current level of evidence
- Vitamin C—An adjunctive therapy for respiratory infection, sepsis and COVID-19
- Overview of the possible role of vitamin C in management of COVID-19
- Repositioning vitamin C as a promising option to alleviate complications associated with COVID-19
- The emerging role of vitamin C in the prevention and treatment of COVID-19
- The long history of vitamin C: From prevention of the common cold to potential aid in the treatment of COVID-19
- Efficacy and safety of vitamin C in the management of acute respiratory infection and disease
- Low level of vitamin C and dysregulation of vitamin C transporter might be involved in the severity of COVID-19 Infection
- A possible application of high dose vitamin C in the prevention and therapy for coronavirus infections
- Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19?
- Ascorbate as prophylaxis and therapy for COVID-19 - Update from Shanghai and U.S. medical institutions
- Vitamin C for COVID-19: A living systematic review
- Is vitamin C an effective agent for the prevention of COVID-19 and treatment of severe infection in the ICU?
- Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)?
- Vitamin C in the treatment of COVID-19
- Feasibility of vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV vitamin C on fatigue
- Therapeutic potential of mega-dose vitamin C to reverse organ dysfunction in sepsis and COVID-19
- The Potential Use of Vitamin C to Prevent Kidney Injury in Patients with COVID-19
- Common anti-oxidant vitamin C as an anti-infective agent with remedial role on SARS-CoV-2 infection. An update
Published commentaries
- Vitamin C for COVID-19 Treatment: Have We Got Enough Evidence?
- Potential benefit of high-dose intravenous vitamin C for coronavirus disease 2019 pneumonia
- Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia
- A Debate on Vitamin C: Supplementation on the Hotline for Critically Ill Patients with COVID-19
- Vitamin C and COVID-19: should clinical trials be prioritized for low-income settings and vitamin C deficient populations?
- Vitamin C as a Possible Therapy for COVID-19
- High-dose intravenous vitamin C may help in cytokine storm in severe SARS-CoV-2 infection
- Role of vitamin C in critically ill patients with COVID-19: is it effective?
- Micronutrient status of COVID-19 patients: a critical consideration
- A new clinical trial to test high-dose vitamin C in patients with COVID-19
- Multi-level immune support by vitamins C and D during the SARS-CoV-2 pandemic
Vitamin C and COVID-19 in the media
There are many articles in the global media regarding vitamin C and COVID-19. Some of the posts directly related to this topic are shown below.
Clinical studies in the media
- Vitamin C could be the secret weapon in the fight against coronavirus
- Vitamin C trials in China podcast
Clinical practice in the media
- Holistic approaches may have reduced coronavirus deaths at this London hospital
- New York hospitals treating coronavirus patients with vitamin C
Case reports in the media
- 'Unusual' IV High-Dose Vitamin C Success Story in COVID-19
- COVID patient with sepsis makes 'remarkable' recovery following megadose of vitamin C
- Richmond doctor shares story of COVID-19 infection, survival
- Emergency room doctor, near death with coronavirus, saved with experimental treatment
Reviews/overviews in the media
- Vitamin C can 'help to prevent severe Covid and speed up recovery'
- Coronavirus update: Vitamin C could save the lives of those severely affected by COVID-19
- Vitamin C can help severe Covid-19 cases, NZ-led review finds
- Vitamin C and COVID-19: A Review
- Vitamin C and COVID-19: Researchers call for status testing and intravenous / oral supplementation
- Vitamin C's effectiveness against COVID may hinge on vitamin's natural transporter levels
- This vitamin may help treat COVID, study finds
- Coronavirus: Vitamin C treatment 'encouraging' says NZ researcher
- Big vote of confidence for Vitamin C as viral fighter. Now being tested
- Is it ethical to keep ignoring the potential of vitamin C to fight the virus?
- Grassroots clinicians pick up on Vitamin C treatment for coronavirus
Podcasts/videos
Feedback
“A useful repository of studies on vitamin C and COVID‑19 is managed by Professor Anita Carr at the University of Otago who has herself has contributed much to research with Vitamin C and both communicable and non-communicable disease.”
Prof R Mithen, Chief Scientist, NZ High Value Nutrition National Science Challenge.
“I live in Canada and I was exposed to COVID‑19 in late December, and fell seriously ill [positive COVID test]. Everyone has a different experience with the virus but what stuck out to me was my constant craving for orange juice, I drank gallons of it, which I usually don't drink. The sickness passed and I stopped craving it. I really think there is something to these studies.”
B Okojie, Canada.
“I feedback quite a lot of information to our Access and Choice practitioners who are seeing a lot of people in primary care with health anxiety. I have highlighted your page to help explain why there is evidence of the use of vitamin C in the treatment of COVID but not as a preventative in place of vaccination.”
H Gibbs, Nutrition Development Advisor, WellSouth Primary Health Network, NZ
Contact
Contact Research Professor Anitra Carr if you wish to provide relevant resources or receive further information.
Email anitra.carr@otago.ac.nz
Tel +64 3 364 0649
How to cite this resource
Carr, A.C. Vitamin C and COVID-19 Research Resource,
otago.ac.nz/christchurch/research/nutrition-in-medicine/vitamin-c