 Discoloured water and yellow precipitate indicate AMD
Discoloured water and yellow precipitate indicate AMD
Acid mine drainage (AMD) is widely acknowledged as one of the greatest environmental problems facing the mining industry. AMD is characterised as having low pH (commonly 2 to 4), leading to elevated concentrations of metals such as cadmium, copper, iron, lead, and zinc.
The seam mined at Wangaloa contains significant organic sulfur and pyrite. As the pyretic sulfur within the coal oxidizes (accelerated by biochemical processes), areas of low pH are produced. Variations of pH, and controls on acidity generation and neutralization over the site are being determined (see below), and in particular the evolution of pH over time during rehabilitation to assess the capacity of the site for natural acid amelioration.
 Michelle Baker sampling water at Wangaloa
Michelle Baker sampling water at Wangaloa
As pyrite weathers, the metals associated with it are also released. Low pH promotes metal leaching from the host rock, and metal mobility increases as hydrogen ions preferentially exchange sorption sites with adsorbed species. Dissolved metals found in slightly elevated concentrations at Wangaloa include arsenic, copper and zinc, all of which have the potential to create toxic conditions unsuitable for plants and invertebrates in closed mine areas. These metals can be mobilized seasonally from the overburden, and pass downstream into wetlands.
There is no evidence of metal toxicity at the Wangaloa site.
Acid water can be generated rapidly in rain events and then dry up soon after the storm. The occurrence of this type of acid drainage is commonly shown up with secondary mineral encrustations on rock surfaces.
The best indicator minerals are: Gypsum, calcium sulphate (CaSO4)

Gypsum crystals (white) as encrustations on pyrite-rich waste rock at Wangaloa
Jarosite, potassium iron sulphate (KFe3[SO4]2[OH]6)
This mineral (below) forms yellow stains on rocks that contain pyrite. The distinctive yellow colour is different from the orange-brown of iron oxyhydroxide (rust) that commonly accompanies jarosite. Both these minerals are visible on the surface of pyrite-bearing Wangaloa coal in the photo below.

Jarosite and iron oxyhydroxide (rust) on pyritic rock
Acid Waters
Groundwater, surface runoff, and the ephemeral surface streams at Wangaloa mine flow into a lake in the centre of the site. The chemical composition of the lake water therefore gives a good indication of the coverage water composition over the site.
The photo below shows this lake, downstream of recently-contoured waste rock, with pyrite-bearing coal. Acid runoff from these surfaces contributed to low lake pH on the early stages of rehabilitation.

Lake at Wangaloa, downstream of recently-contoured waste rock, with pyrite-bearing coal
Regular monitoring of the lake water pH has showed that there has been a general rise in lake pH (see graph belwo).
Fluctuations can occur on a 1-2 month time scale. However, the lake pH has been near 6 for most of the latter stages of rehabilitation. Some lime was added to the lake in 2002 to try to raise the pH initially.
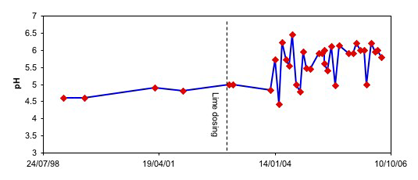
Lake water pH at Wangaloa
Boron in water
Coal very commonly contains naturally elevated amounts of the trace element boron. The boron typically comes from seawater affecting coastal swamps from which the coal is derived. This boron can be dissolved in rainwater and ground water that flows past coal-rich waste rock. Hence, Wangaloa mine waters can contain elevated levels of boron. The graph below shows typical boron levels in the lake.
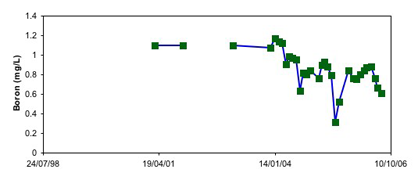
Lake water Boron at Wangaloa
The boron levels are slowly declining. Boron can be toxic to freshwater organisms, especially fish, so low boron levels are desirable. However, boron is also an important micronutrient, and if boron levels are too low in water, organisms will "starve". Boron levels near to about 1mg/L appear to be acceptable for aquatic organisms.
Related
Closed Coal Mine - Wangaloa Coal Mine
- Introduction
- Acid Mine Drainage and pH
- Soils
- Revegetation
- Invertebrates
- Natural Development of ecosystems
- Water Quality
- Bibliography
