

Associate Professor Vernon Ward
He and his colleagues are using the empty shell of the rabbit haemorrhagic disease virus to tailor an immune response against cancer.
Having decimated New Zealand's rabbit population in the 1990s, the virus underlying rabbit haemorrhagic disease (RHD) is showing great promise as a tool in the fight against cancer.
A multidisciplinary research team at Otago is developing ways of bioengineering the empty shell of the virus – known as virus-like particles (VLPs) or viral nanoparticles – and using them to stimulate the body's immune system against cancer.
The team includes virologist Associate Professor Vernon Ward and immunologists Associate Professor Margaret Baird (Department of Microbiology and Immunology) and Dr Sarah Young (Department of Pathology).
Ward's focus is on producing the viral shell – and plenty of it. This is done by inserting the gene from the RHD virus that encodes for the capsid, or shell protein, into an insect baculovirus.
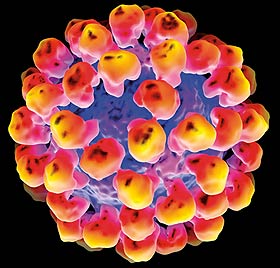
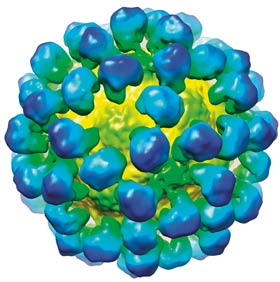
Images 1 and 2
3D representation of the rabbit haemorrhagic disease (RHD) virus-like particle.

Image 3
Cross-section through a RHD virus-like particle showing the protein shell used to carry tumour-associated antigens for vaccination purposes
“We grow that baculovirus in a broth of cells and, when it infects and replicates in those cells, it produces the RHDV capsid protein we want,” says Ward. “That automatically assembles into the shell or viral nanoparticle that we then purify.”
At that point the scientists have the empty shell of the RHD virus which they use as “a versatile scaffold”.
“We can modify the proteins, we can chemically couple things to them – we use them as a carrier. That's what it's about,” says Ward.
“We can modify the unit – we can take the 180 protein copies that make up the shell of the virus and modify it, or we can pull that shell apart and then put it back together in the test tube, so we can put things inside that shell and use it as a vessel.”
For example, they can attach tumour-associated proteins inside the shell to stimulate the cancer immune response to specifically target that cancer. They can also add molecules to the outside of the particle to generate an antibody response.
“By packaging molecules on the inside you have a delivery system. By attaching molecules to the outside you can target specific cell types and specific pathways to stimulate the immune system to get a better response.”
Ward says the multidisciplinary approach has been valuable. As a virologist he has been able to develop the materials, while his immunologist colleagues can see if what they make actually produces the desired immune response.
The group has also been working with a local oncologist to get an idea of what would be feasible and practical in a clinical setting.
Ward says they chose the RHD virus because it generates a particularly good immune response and the type of immune response needed to target a particular type of cancer cell and clear a tumour. They also wanted to avoid a pre-existing immune response to the carrier.
“One of the things we wanted was a VLP, or viral nanoparticle, that would mean no carrier immunity when you started the treatment. This is a rabbit virus, so rabbits might have some immune response to it, but people don't.
“If we did have pre-existing immunity to the carrier, you are going to run the risk that the system is going to react to the carrier more than the epitope you really want it to react to. We want that ability of the carrier to stimulate an immune response, but we don't want the tumour antigen to be hidden in that process.”
Ward says the eventual goal will be to be able to take particular proteins which are unique to the tumour a particular person is suffering from and attach them to a viral nanoparticle and reintroduce it so the body makes a response specific to that tumour.
“It then starts to become personalised medicine.”
Funding
- Health Research Council
- Cancer Society of New Zealand
- University of Otago
- Otago Medical Research Foundation
- Lottery Health Research
