Congratulations to Otago Biochemist Professor Kurt Krause and his team of researchers who have just secured an HRC Project Grant for their research into a rapid tuberculosis cure.
Tuberculosis remains a huge public health problem. Over two billion people worldwide are infected with the Mycobacterium tuberculosis bacteria that causes the disease, and more than one million die of tuberculosis each year.
Although we have had antibiotics that treat tuberculosis for decades, they are slow acting, requiring many months of intensive pill-taking. The rise of extensively drug-resistant strains makes the future even more bleak on the tuberculosis front, threatening to return us to the pre-antibiotic era. New drugs for the rapid treatment of tuberculosis would be a significant help in fighting the disease.
Our scientists are steadily working on a new drug solution.
One of the challenges of tuberculosis bacteria is that they can hide inside a person's body without causing symptoms, sometimes for many years, before they eventual cause disease. When they are hiding in this 'latent' state, they are particularly hard to treat with antibiotics.
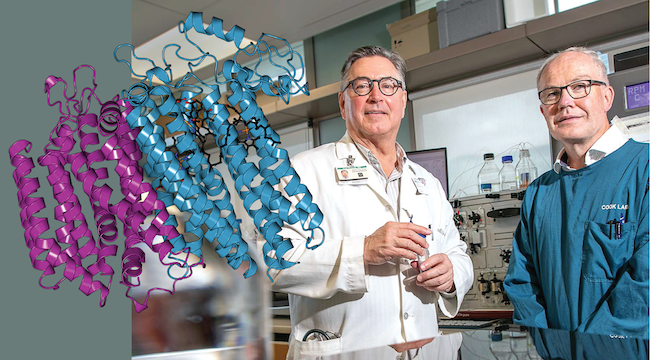
A gamechanger piece of research showed that a protein called bd oxidase allows tuberculosis bacteria to hide in this latent state.
Blocking the actions of the bd oxidase protein at the same time as a patient is treated with additional anti-tuberculosis therapy could make treatments much quicker and more effective.
Two years ago, Professor Kurt Krause, Professor Greg Cook (Department of Microbiology and Immunology), their research teams, and collaborators from the Max Planck Institute of Biophysics, Germany, figured out the exact atomic structure of the bd oxidase protein and published it in the journal Nature Communications.
This structure, along with the HRC Project Grant of $1.2 million over three years, will allow Kurt, Greg and an expanded team of local and international collaborators to move forward on their goal.
They aim to use the precise atomic details of the bd oxidase structure to guide their search for a molecule that will bind and block the protein and can be developed into a new tuberculosis drug.
Ka mau te wehi! Outstanding work! And we wish Kurt, Greg and colleagues the best on their upcoming research journey.
Image above: From left, bd oxidase, Prof Kurt Krause and Prof Greg Cook (photo from University of Otago Magazine).
Protein illustrated using Protein Imager.