What is the likelihood of a COVID-19 vaccine being developed and how long will the process take? Professor David Murdoch, Dean of the University of Otago, Christchurch, addressed these questions in a recent online summit.

Professor David Murdoch.
Professor Murdoch has experience working in vaccine evaluation and deployment and understands that at least 35 vaccine candidates are entering various stages of development around the world.
“The fact clinical trials are starting is amazing when you think that four months ago we didn't even know this virus existed. So that is quite extraordinary.
“But it's important to put into context, we're still a way from having an effective vaccine. By the best estimates we're still 12-18 months away even if everything went well.”
That may seem a long way in the future but it's worth remembering past vaccines have taken 10-15 years to get to market.
“I'm optimistic. There's a lot of clever people working on this.”
The six-phase process to develop a vaccine:
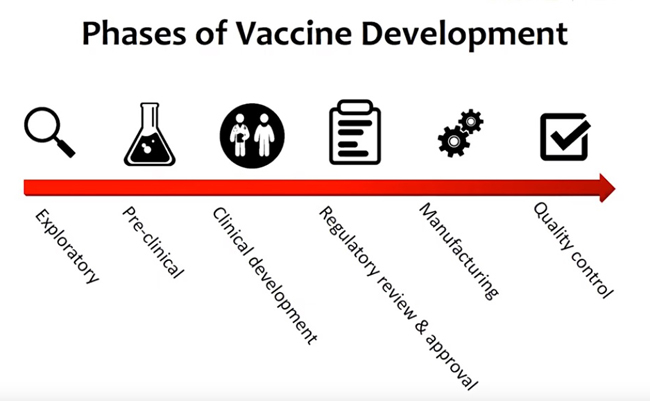
Exploratory
This phase is where scientists come up with ideas of how to target the vaccine. In the case of COVID-19 information about the structure of the virus was released very quickly.
Pre-clinical
Once you've got the ideas you want to know: Is it going to work? Is it going to be harmful? Live cells will be grown in the lab or perhaps some animal studies looking at whether it will produce an immune response. There's a lot of information gathered at this time.
Clinical development
A lot of vaccines fail at the pre-clinical stage but if a vaccine looks promising it enters this phase. This stage is about really determining: Is it safe, will it work and what doses should be used? It's a big phase which requires a lot of funding. The process is very tightly controlled with regulatory bodies involved very early on.
Usually it's broken into three sections. Phase 1 is typically less than 100 healthy volunteers. The goal is to get an idea of the development of the immune response. If it passes that it will go to Phase 2 study, which is the order of hundreds of healthy participants. It's a very similar process looking again at the immune response but getting a better idea about dosage. Phase 3 studies involve thousands or maybe tens of thousands of volunteers.
A typical design would be a placebo-controlled trial, comparing the vaccine to a placebo. It's looking over time at who develops the infection. Once the study is finished you look at the proportion who get COVID-19 among those who got the vaccine and among those who didn't get the vaccine.
Hopefully with a vaccine that worked there would be very little if any COVID-19 among those who are vaccinated and you'd see a number of cases among those who'd received the placebo. So these studies will be located where there is ongoing transmission of COVID-19. Ultimately at this stage we'll know: is it safe? Does it work? What sort of doses can be given?
Regulatory review and approval
It will then go through a series of checks with the various regulators making sure it meets all the requirements for an effective and safe vaccine.
Manufacturing
The vaccine will then progress to manufacturing. This will almost always involve industry with the ability to mass produce. Thought needs to be given to that very early on. There's no point developing the vaccine if you can't make enough.
Quality control
This is about knowing that it's a vaccine that can be stored and delivered and is having the impact it should be having. So ongoing monitoring is critical.

The development of the vaccine is not the end of the process.
Each country or region will have its own challenges and work is required to determine the suitability of the vaccine for the population along with funding and logistics considerations.
“Equity and inequity are issues. Once a vaccine is developed will it be going to all countries and all areas that need it?”
Professor Murdoch says the Coalition for Epidemic Preparedness Innovations, established in 2015 to help fund vaccines and other innovations, will work to ensure the equity issue is covered.
It was very difficult to say how long it might take to make a distribute a successful vaccine.
“But I'm constantly surprised by how quickly things have come along. Ebola really pushed on the technology. Hopefully we'll see a fairly quick process.”
