OMNI's image gallery brings together images University of Otago staff, researchers, and students have created using microscopy techniques.
Visit Our people to read more about OMNI staff's specialist interest areas.
Rebecca Campbell
Associate Professor Rebecca Campbell, Centre for Neuroendocrinology, is interested in defining how the gonadotrophin releasing hormone (GnRH) neurons, well established as the ultimate downstream regulators of the central control of reproductive function, are regulated by the multitude of external and internal cues necessary for successful fertility.
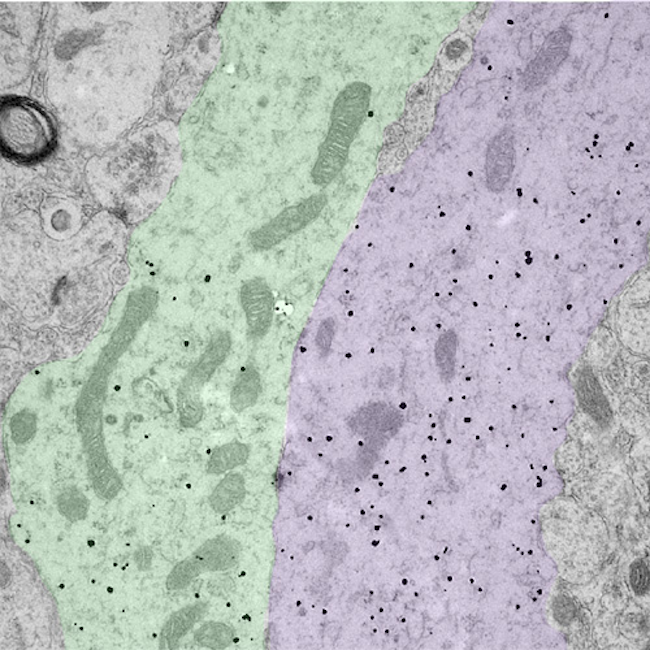
Associate Professor Rebecca Campbell: Image of bundled GnRH neuron dendrites.
The small black dots label the dendritic arms of two GnRH neurons (pseudocolored green and purple), cells in the brain that control fertility. The discovery that these two distinct dendritic elements, cut in the longitudinal plane, are found in close apposition and possess an attachment plate (shaded zone) between them, is suggestive of how these scattered cells communicate with one another.
Gemma Cotton
Dr Gemma Cotton is a Research Fellow with the Sir John Walsh Research Institute, Faculty of Dentistry, at the University of Otago.
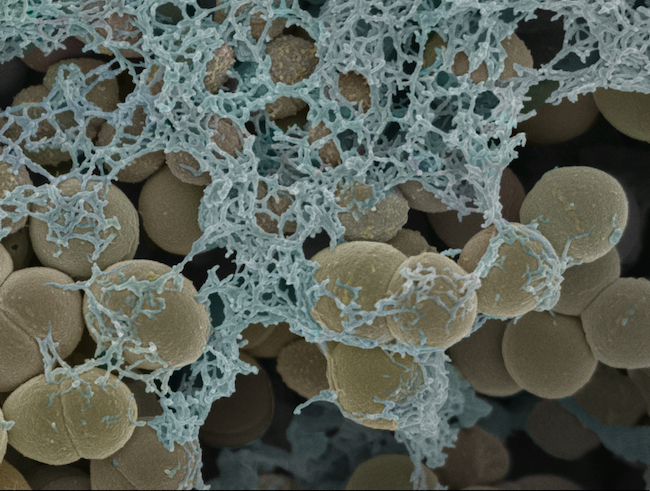
Gemma Cotton: Streptococcus gordonii biofilm grown for in vitro biofilm testing of novel bacterial gels.
Preparation of the sample required the invaluable sample preparation knowledge of Liz Girvan, where we dehydrated the sample through a series of ethanol washes and used critical point drying to maintain the structural integrity of the cells.
We used secondary electron imaging on the JEOL 6700F FESEM which provided high-resolution imaging of the fine surface morphology and details of the extracellular matrix of the biofilm. The images permitted fine details to be monitored and compared across a series of variables.
Liz Girvan
Liz Girvan is a member of OMNI's Electron microscopy team. Her expertise is in Scanning Electron Microscopy (SEM). These three images were submitted for the 2018 School of Biomedical Sciences photo competition.
Email liz.girvan@otago.ac.nz
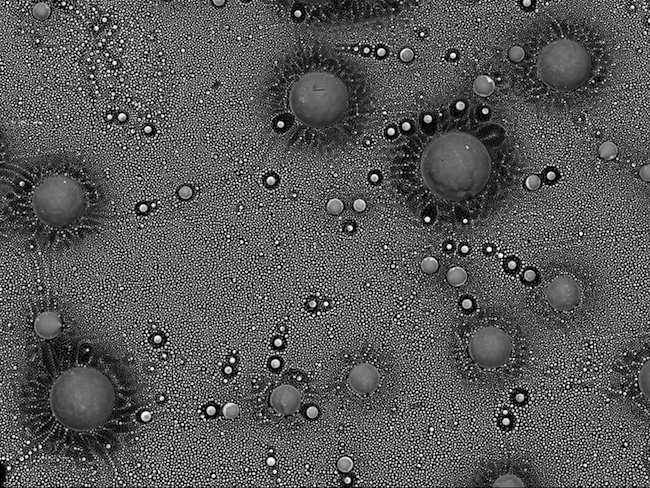
Liz Girvan: Outer space.
This space-like scene is from a small area inside the glass from a blown fuse. The fuse came from a power supply that ran part of the same microscope that I used to take the image. It was taken on a JEOL 6700F Scanning Electron Microscope.
The image was taken using a backscatter detector. This detector is used to show any compositional differences within the sample. The little spheres are made up of copper and lead. They have come from the metal wire in the fuse.
They show up brighter than the darker glass background as they are higher in atomic number.
This image came about as I was curious what the inside of the blown fuse would look like. I didn't expect to see something so beautiful from a fuse that had caused me so many problems with my microscope!

Liz Girvan: Trapped.
I was searching a plant sample for something completely different when I came across this spider's web. Trapped in the spider's web was this tiny insect. The sticky strands of the web have also trapped bits of dust and pollen. It was taken on a JEOL 6700F Scanning Electron Microscope.

Liz Girvan: Waterfall (this image won the professional staff section of the 2018 School of Biomedical Sciences photo competition).
I am actually not sure what this wee structure is. It looked to me like some kind of frozen waterfall, it really stood out amongst a sea of mineral crystals. It's pretty small – about the width of a human hair.
It was inside a small piece of limestone I picked up while on a walk on the family farm. I am always picking up bits and pieces to look at under the SEM. Looking at unknown samples is a really good way of trying new techniques on the SEM. Also, you always find amazing and beautiful structures!
Uwe Kaulfuss
Dr Uwe Kaulfuss is a postdoctoral fellow and teaching fellow with the Department of Geology.
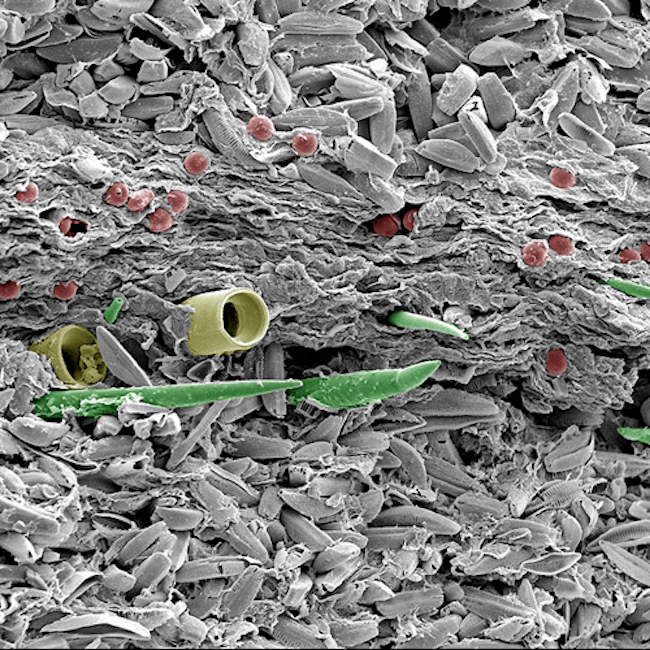
Uwe Kaulfuss: Image of volcanic lake sediment.
This image demonstrates secondary electron imaging, and can be performed on our Zeiss Sigma VP FEG SEM, JEOL FE-SEM6700, Cambridge 360 SEM (up to 150,000 sample dependent).
Uwe's image shows finely-layered sediment from a 23 million year old volcanic lake in Middlemarch, Otago. Light-coloured summer layers of pennate diatoms are interbedded with a winter layer of plant debris, centric diatoms (yellow), freshwater sponges (green), and algal resting spores (red). Colours in this scanning electron microscope image are artificial.
Christine Jasoni
Associate Professor Christine Jasoni's research is on discovering how the maternal environment during pregnancy affects the formation of the fetal brain. Her team uses an array of techniques including molecular and cellular biology, mouse genetic models, live cell imaging, and bioinformatics to address their scientific questions. Christine has shared three confocal microscopy images of the brain.
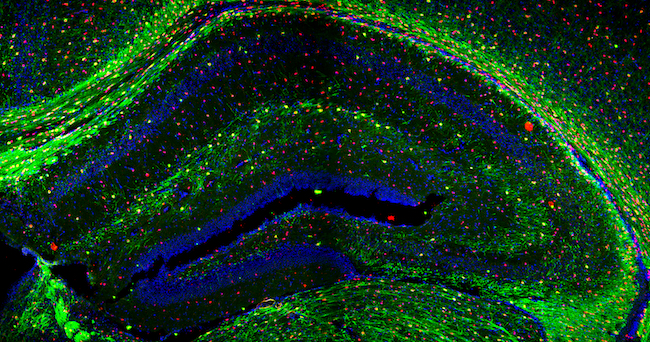
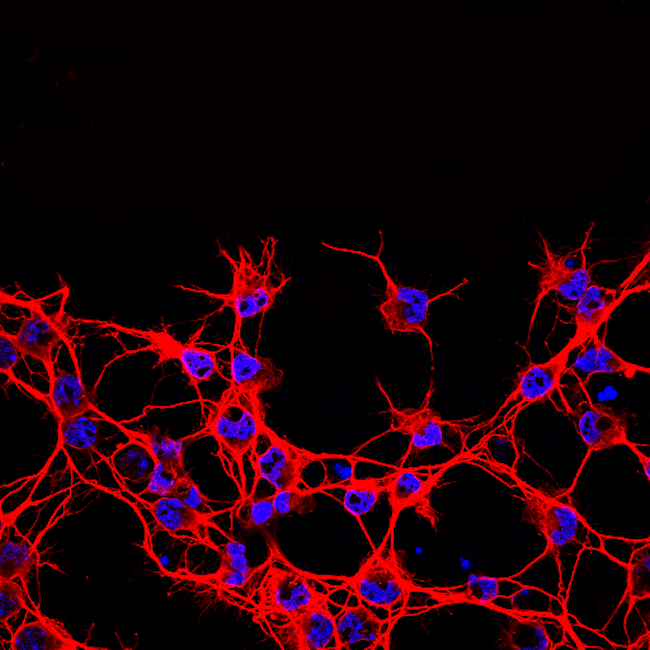
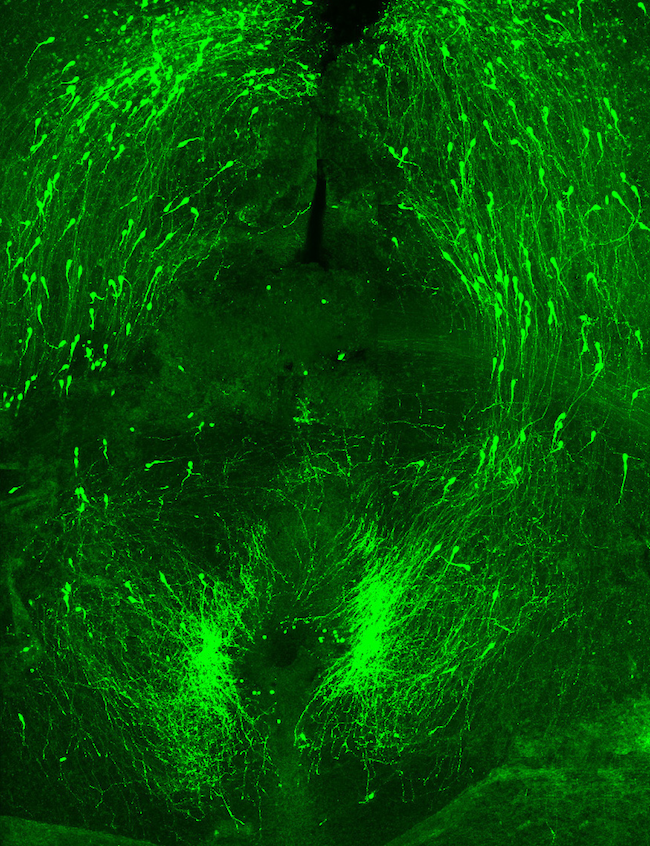
Sharon Lequeux
Sharon Lequeux is a member of OMNI's Electron Microscopy team. These two images were submitted for for the 2018 School of Biomedical Sciences photo competition.
Email sharon.lequeux@otago.ac.nz
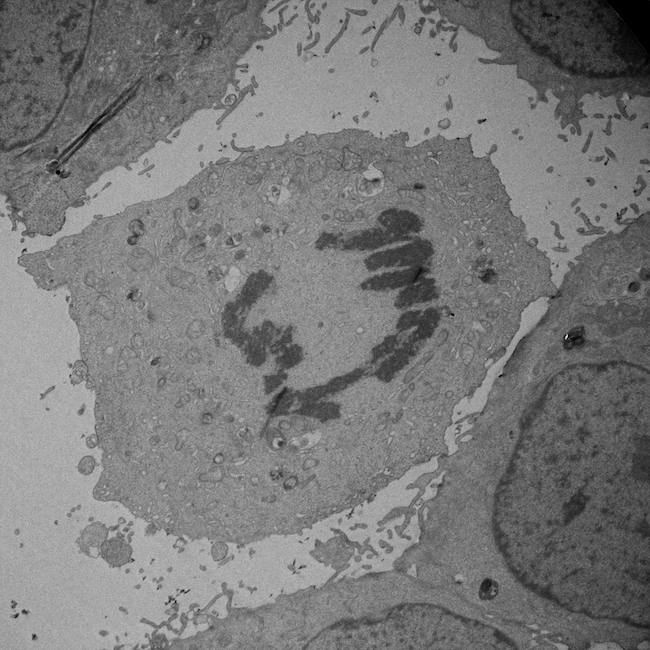
Sharon Lequeux: Lung cancer cell undergoing mitosis.
TEM micrograph taken on a JEM-2200FS Cryo-TEM at 2000x magnification. The chromosomes have condensed and the sister chromatids will be pulled towards opposite ends of the cell.
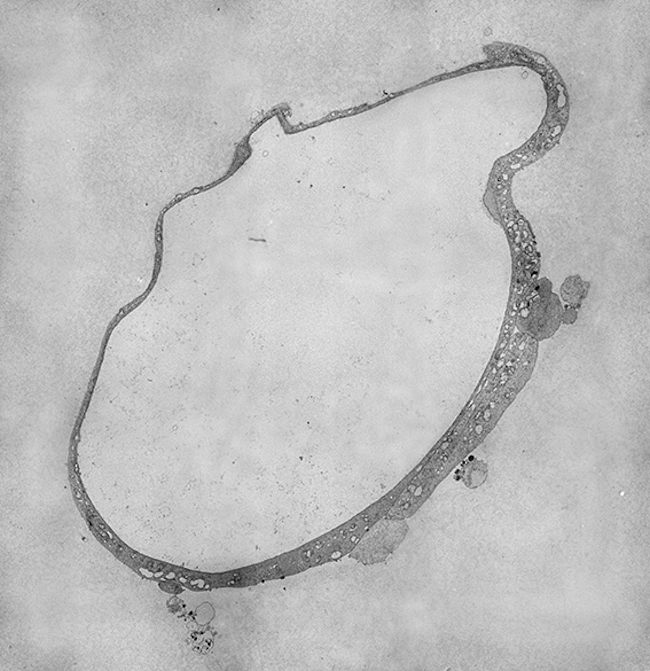
Sharon Lequeux: Gut organoid.
A four-day old mini-gut cultured from an intestinal stem cell. As they age these organoids grow complex structures in culture that closely parallel their in-vivo counterparts. Tissue provided by the Butt Lab (Microbiology). Micrographs were taken at 2500x on a JEM2200FS Cryo-TEM and montaged using IMOD and Photoshop.
Andrew McNaughton
Andrew McNaughton was an OMNI staff member who works with confocal and light microscopes, and the X-ray µCT. Consequently, he has developed a diverse range of interests and is developing more of the image analysis aspects of data acquisition from these instruments. He also uses 3D reconstruction of sometimes large data sets.
Confocal microscopy in particular is capable of producing some remarkable images.
Email confocal.microscopy@otago.ac.nz
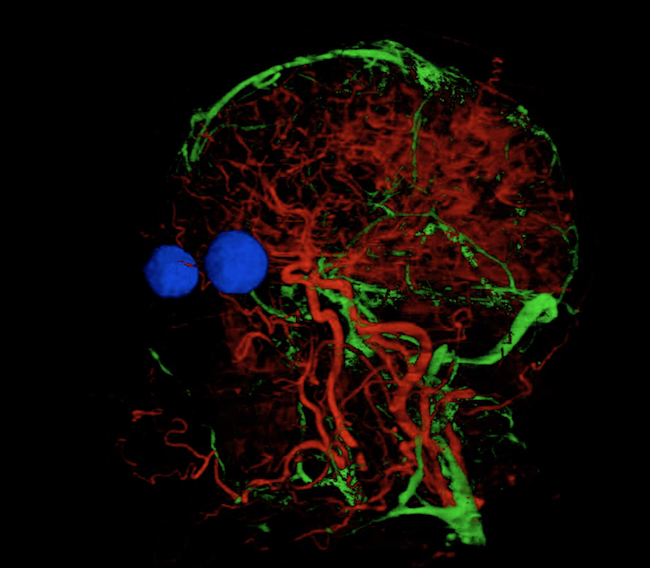
Andrew McNaughton: Look head (this was another staff entry in the School of Biomedical Sciences photo competition).
The veins and arteries were selected from a series photographs of horizontal slices taken through a human head. Rather than laboriously tracing each one, open source machine learning software (Ilastik) was taught to recognise the veins and arteries. The software then traced through all the slices to reveal the network of vessels running through the brain and parts of the face. The eyes were added in a similar manner to help show the overall context.
This is a single frame from a 3-dimensional model which can be rotated to reveal the network of vessels from any angle.
David Prior
Professor David Prior, Department Of Geology, has research interests spanning from the understanding of the material processes that control behaviour of crystalline materials (including rocks, industrial ceramics, metals and ice), to the large-scale tectonic and thermo-chemical processes that control the evolution of the Earth's interior.
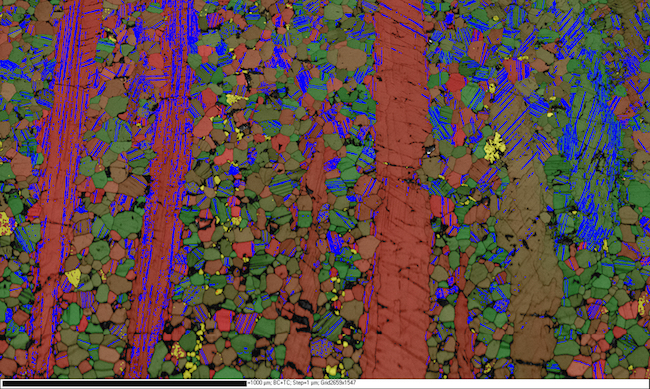
David Prior: Image of sheared rock.
This image is an electron backscatter diffraction (EBSD) map of a sheared rock made mostly of grains of the mineral plagioclase. The red to green colour of each pixel shows the orientation. Yellow points are a different mineral (hornblende). Tone underlying colour shows changes in EBSD pattern quality. Blue lines are twin boundaries, where the crystal axes change by 180 degrees.
Electron backscatter diffraction imaging can be performed on our Zeiss Sigma VP FEG SEM.
